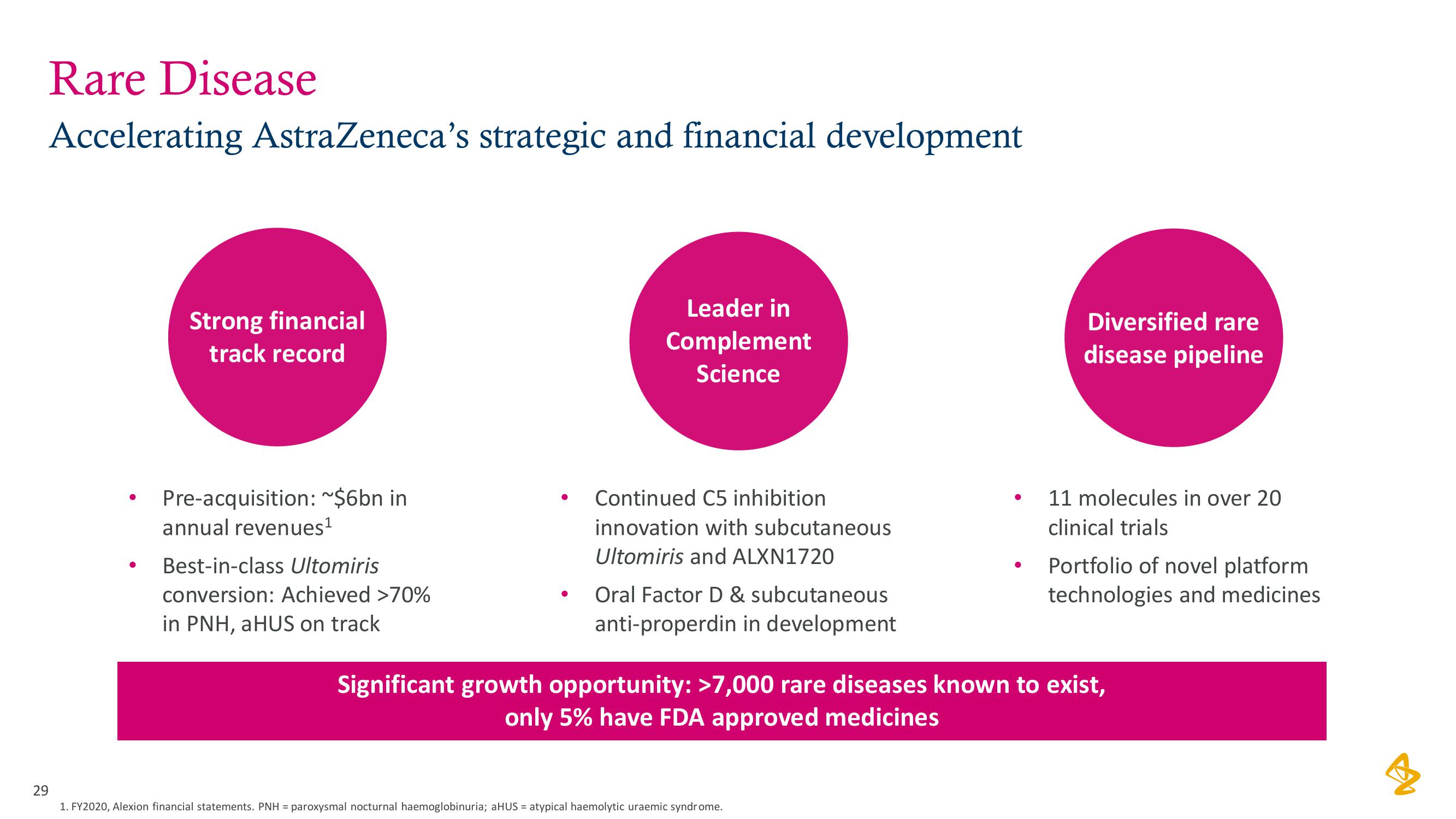
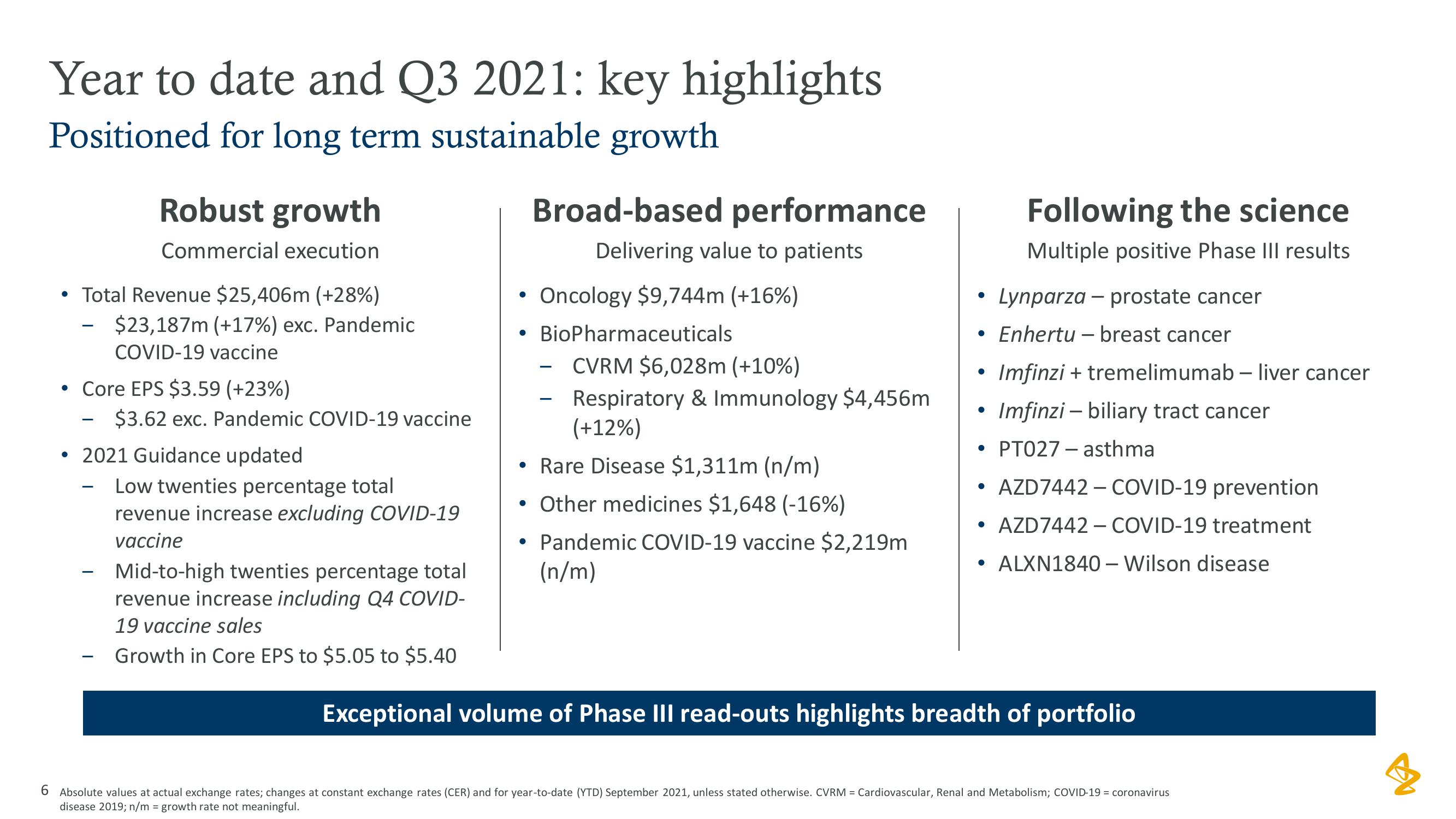
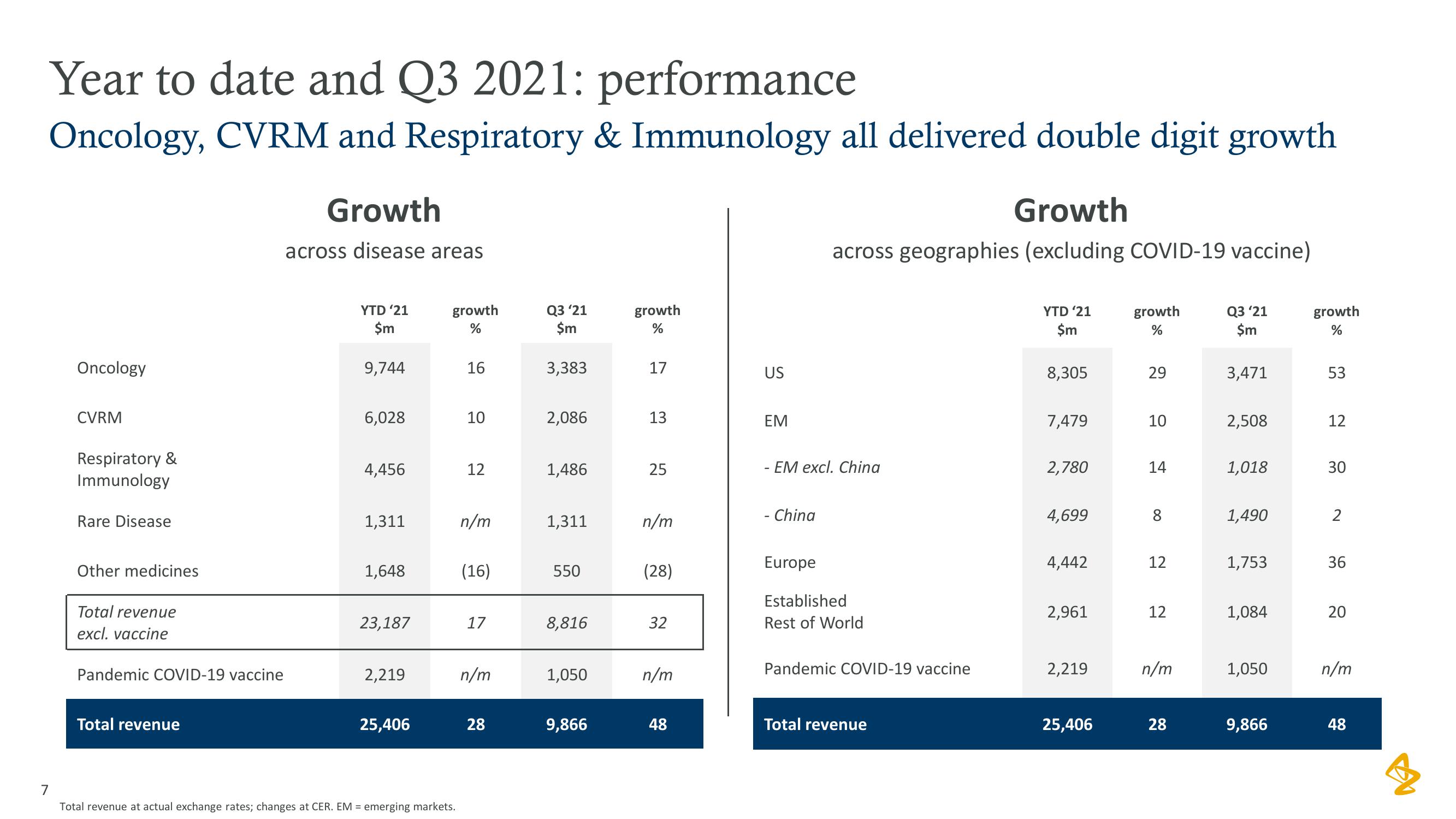
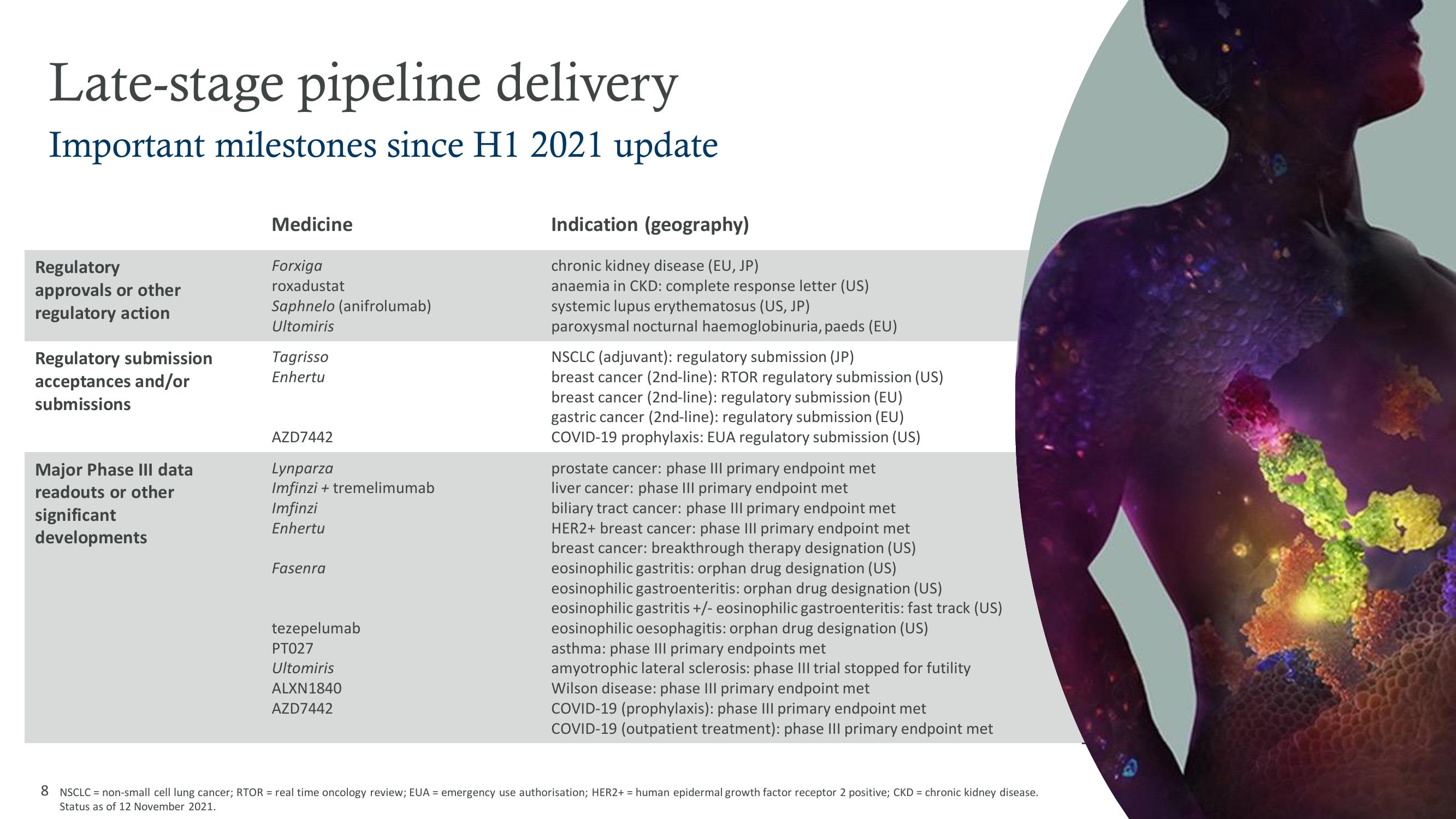
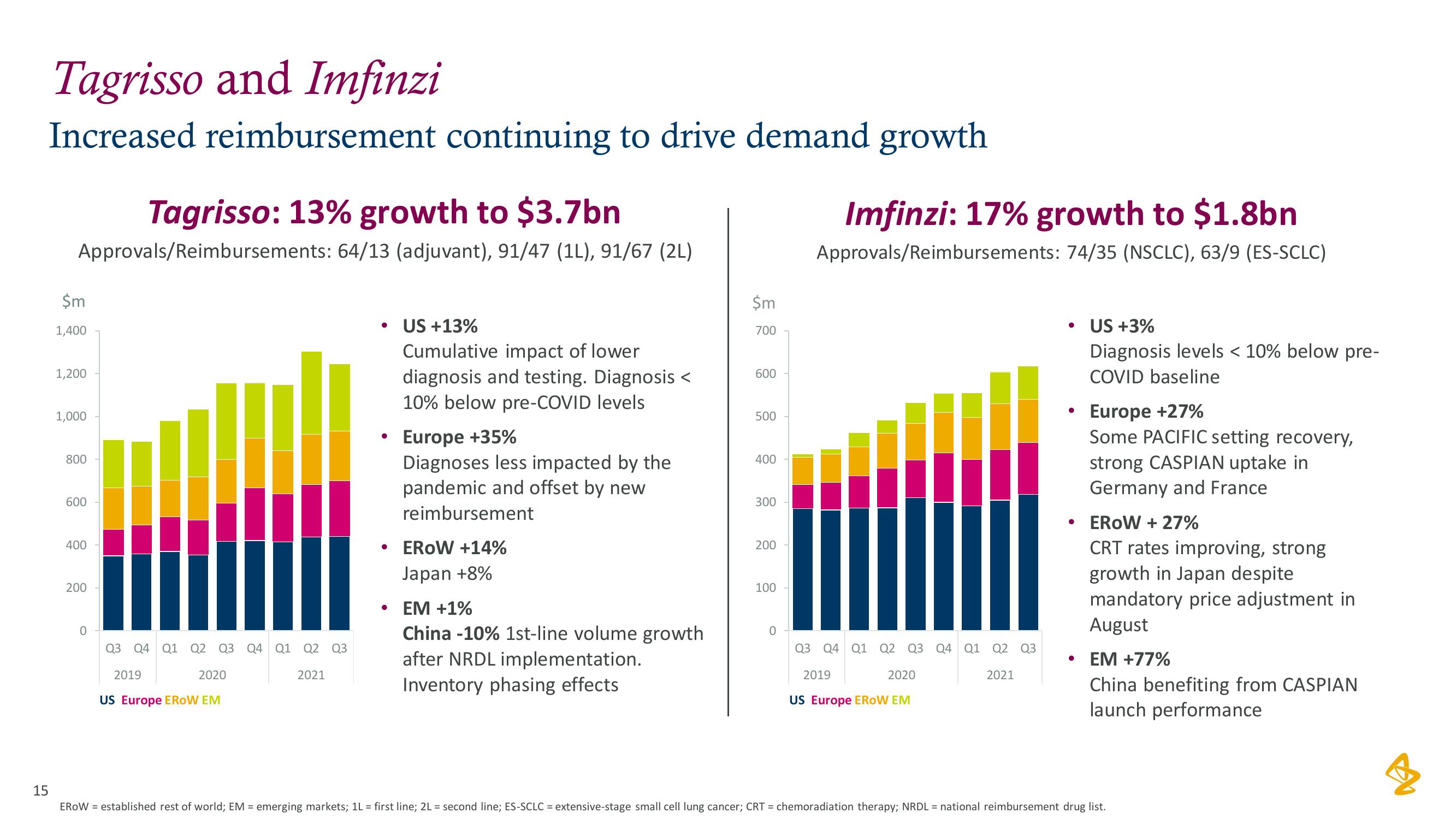
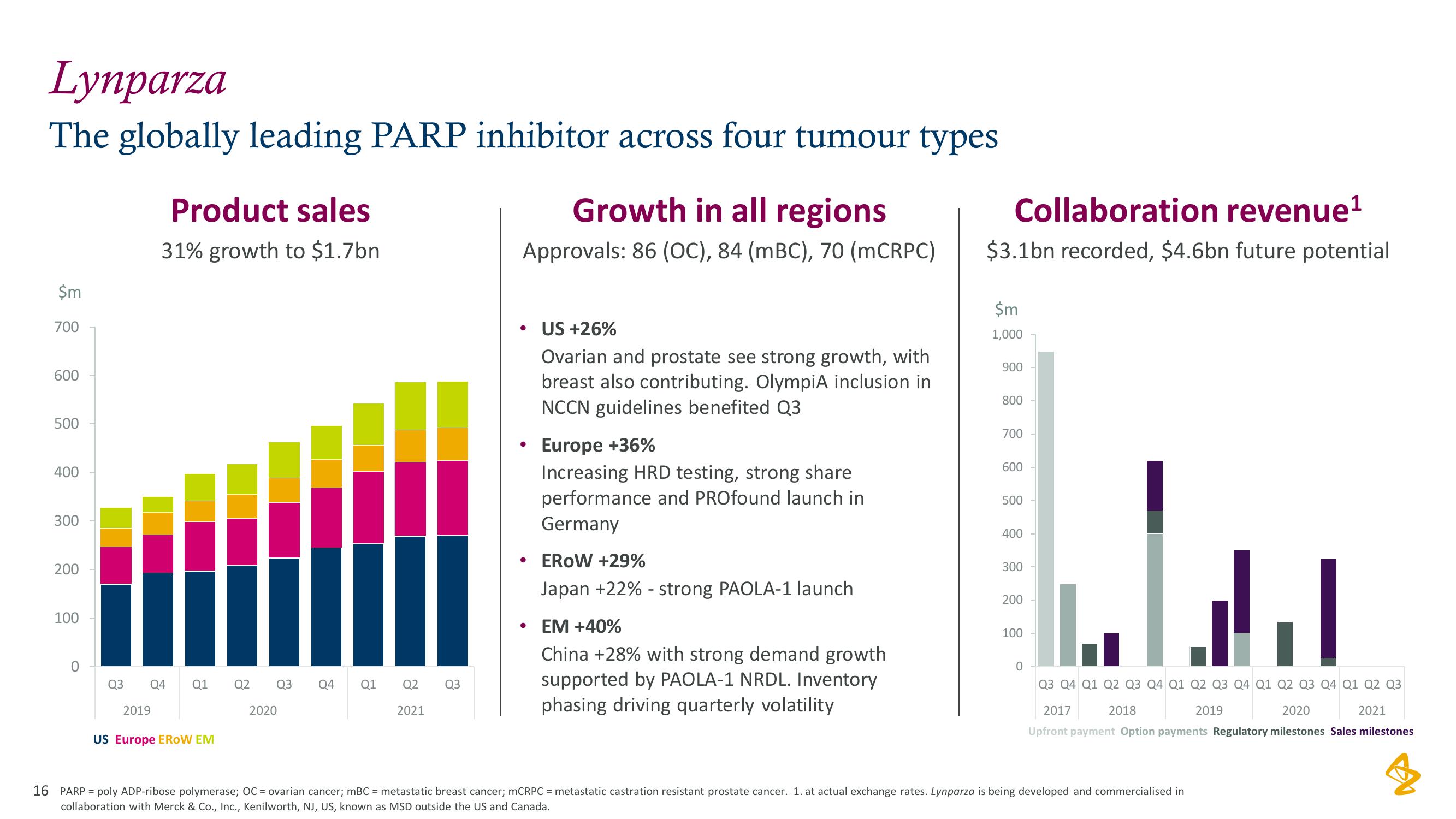
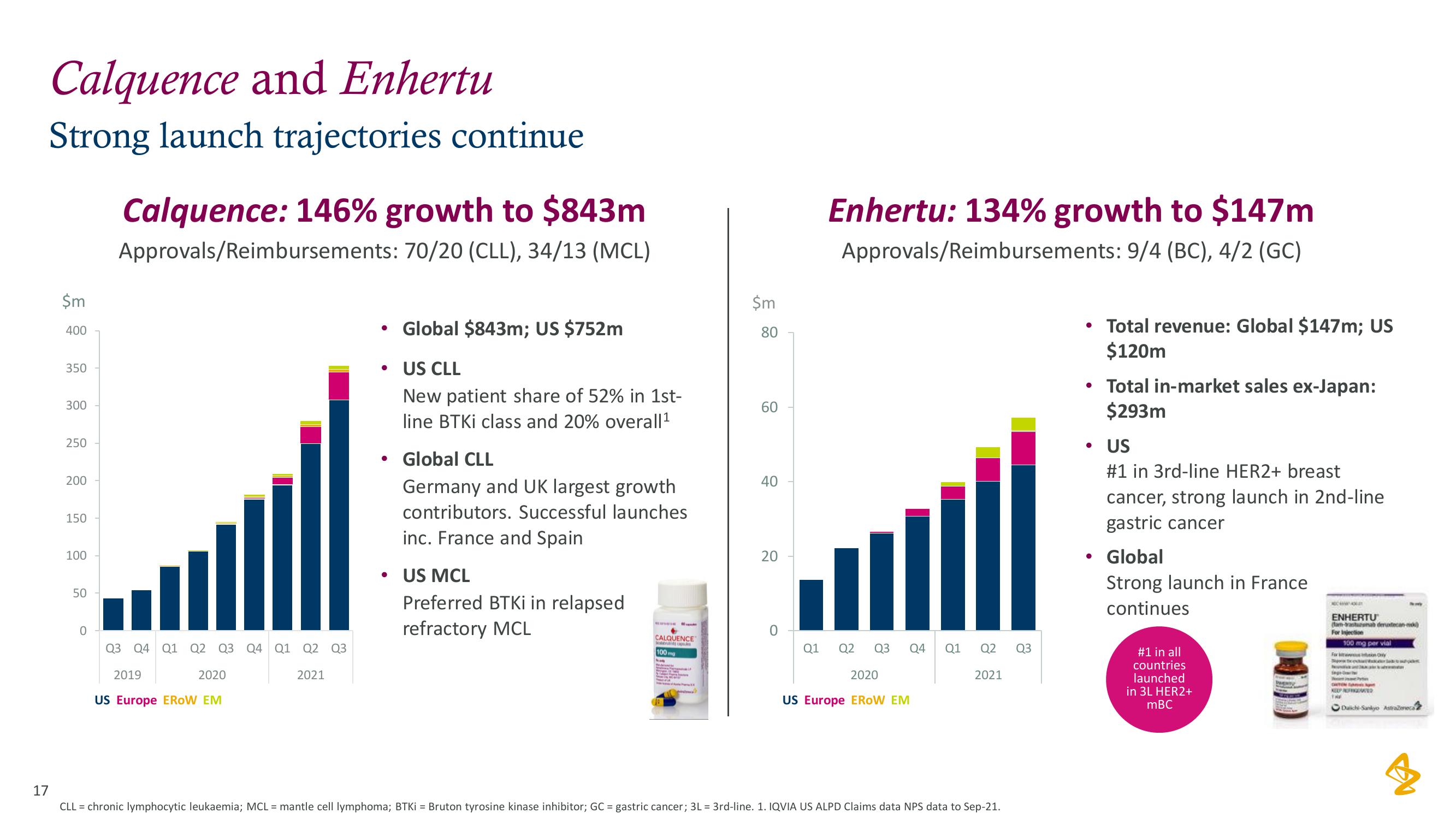
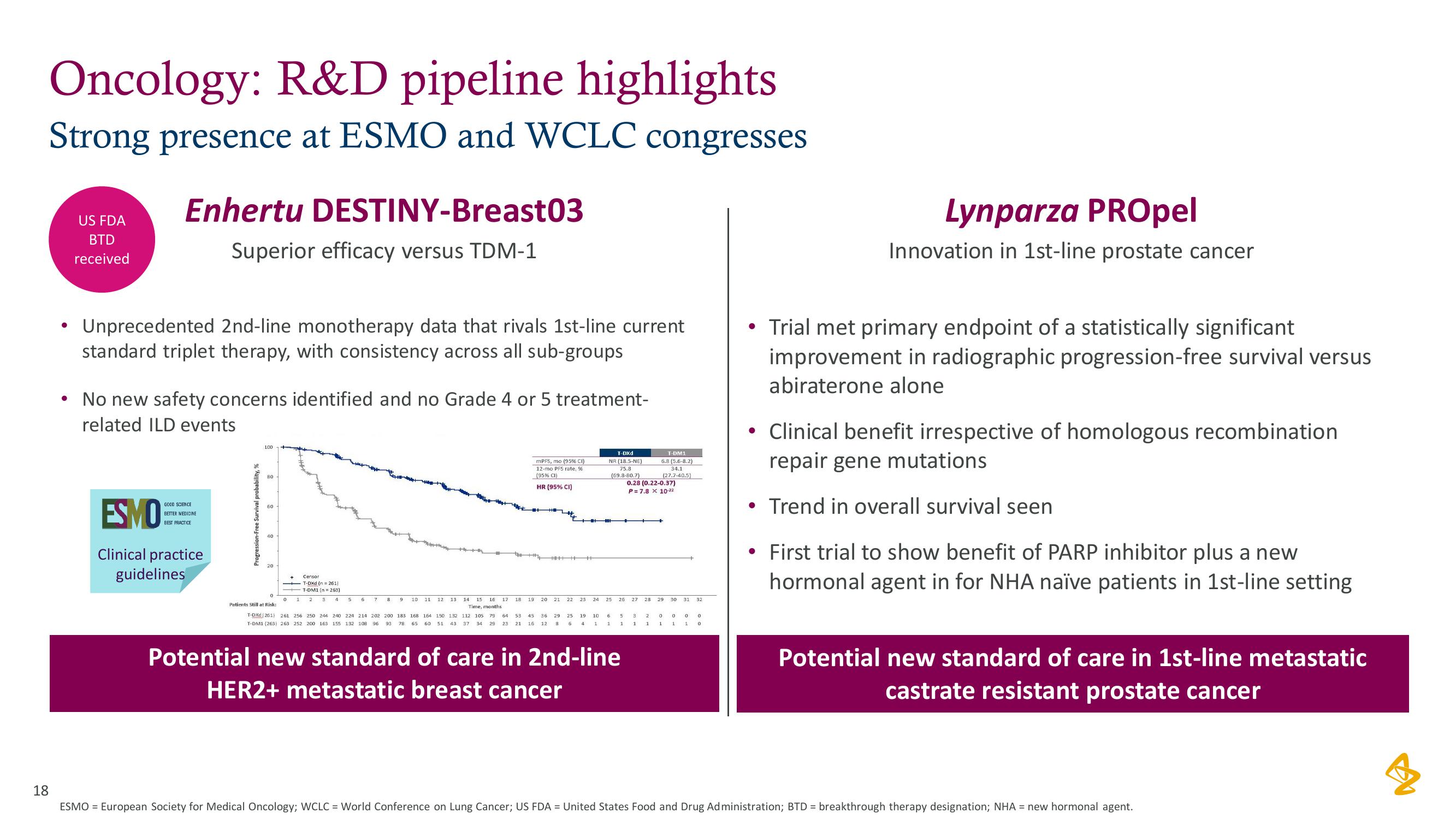
AstraZeneca Results Presentation Deck
Made public by
Astrazeneca
sourced by PitchSend
Creator
astrazeneca
Category
Healthcare
Published
November 2021
Slides
Transcriptions
Download to PowerPoint
Download presentation as an editable powerpoint.
Related