AstraZeneca Investor Day Presentation Deck
Baseline characteristics
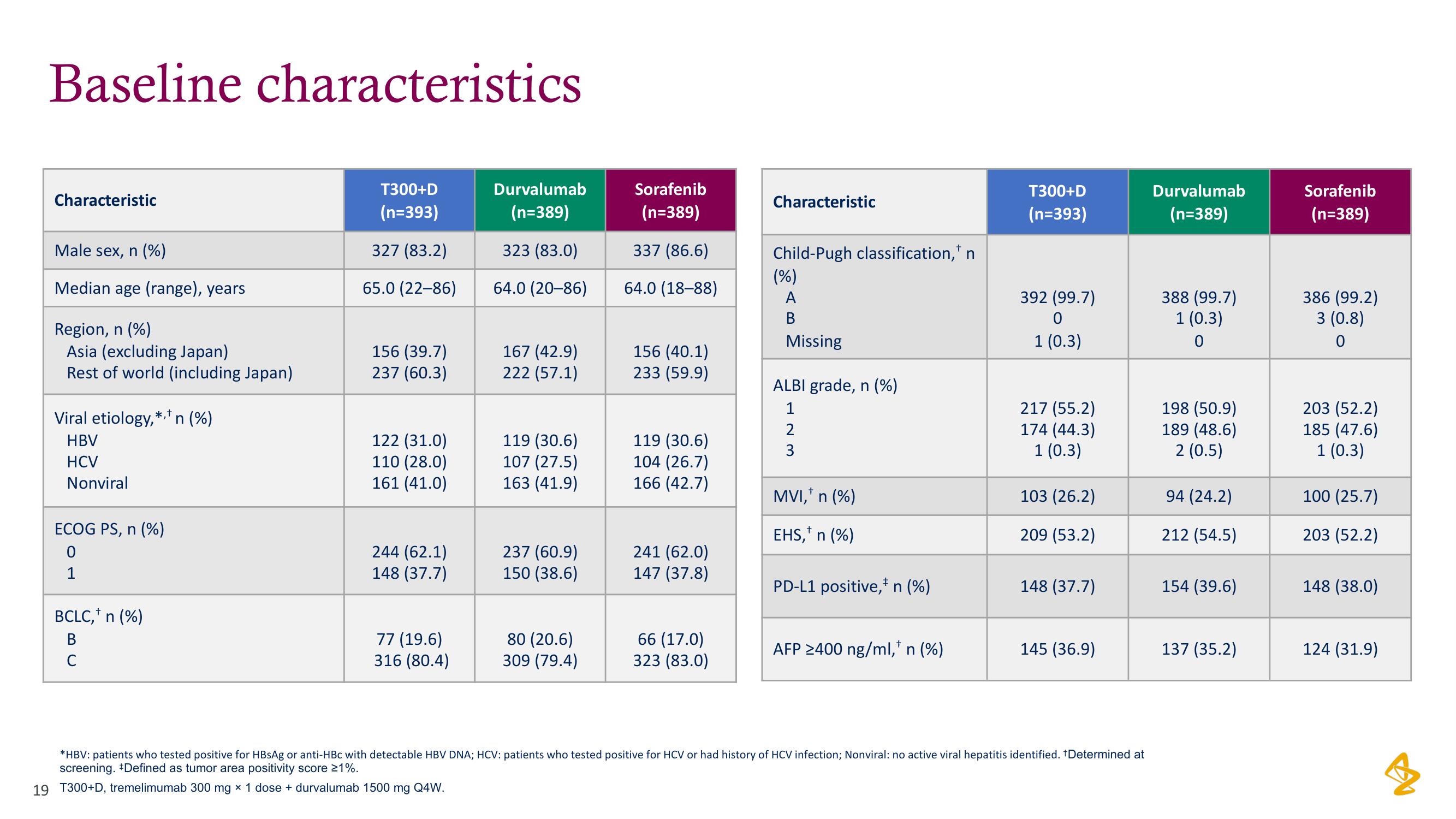
Characteristic
Male sex, n (%)
Median age (range), years
Region, n (%)
Asia (excluding Japan)
Rest of world (including Japan)
Viral etiology,*,* n (%)
HBV
HCV
Nonviral
ECOG PS, n (%)
0
1
BCLC, n (%)
B
C
T300+D
(n=393)
327 (83.2)
65.0 (22-86)
156 (39.7)
237 (60.3)
122 (31.0)
110 (28.0)
161 (41.0)
244 (62.1)
148 (37.7)
77 (19.6)
316 (80.4)
Durvalumab
(n=389)
323 (83.0)
64.0 (20-86)
167 (42.9)
222 (57.1)
119 (30.6)
107 (27.5)
163 (41.9)
237 (60.9)
150 (38.6)
80 (20.6)
309 (79.4)
Sorafenib
(n=389)
337 (86.6)
64.0 (18-88)
156 (40.1)
233 (59.9)
119 (30.6)
104 (26.7)
166 (42.7)
241 (62.0)
147 (37.8)
66 (17.0)
323 (83.0)
Characteristic
Child-Pugh classification, n
(%)
A
B
Missing
ALBI grade, n (%)
1
2
3
MVI, n (%)
EHS,+ n (%)
PD-L1 positive, n (%)
AFP ≥400 ng/ml,* n (%)
T300+D
(n=393)
392 (99.7)
0
1 (0.3)
217 (55.2)
174 (44.3)
1 (0.3)
103 (26.2)
209 (53.2)
148 (37.7)
145 (36.9)
*HBV: patients who tested positive for HBsAg or anti-HBc with detectable HBV DNA; HCV: patients who tested positive for HCV or had history of HCV infection; Nonviral: no active viral hepatitis identified. *Determined at
screening. *Defined as tumor area positivity score 21%.
19 T300+D, tremelimumab 300 mg x 1 dose + durvalumab 1500 mg Q4W.
Durvalumab
(n=389)
388 (99.7)
1 (0.3)
0
198 (50.9)
189 (48.6)
2 (0.5)
94 (24.2)
212 (54.5)
154 (39.6)
137 (35.2)
Sorafenib
(n=389)
386 (99.2)
3 (0.8)
0
203 (52.2)
185 (47.6)
1 (0.3)
100 (25.7)
203 (52.2)
148 (38.0)
124 (31.9)
$View entire presentation