AstraZeneca Results Presentation Deck
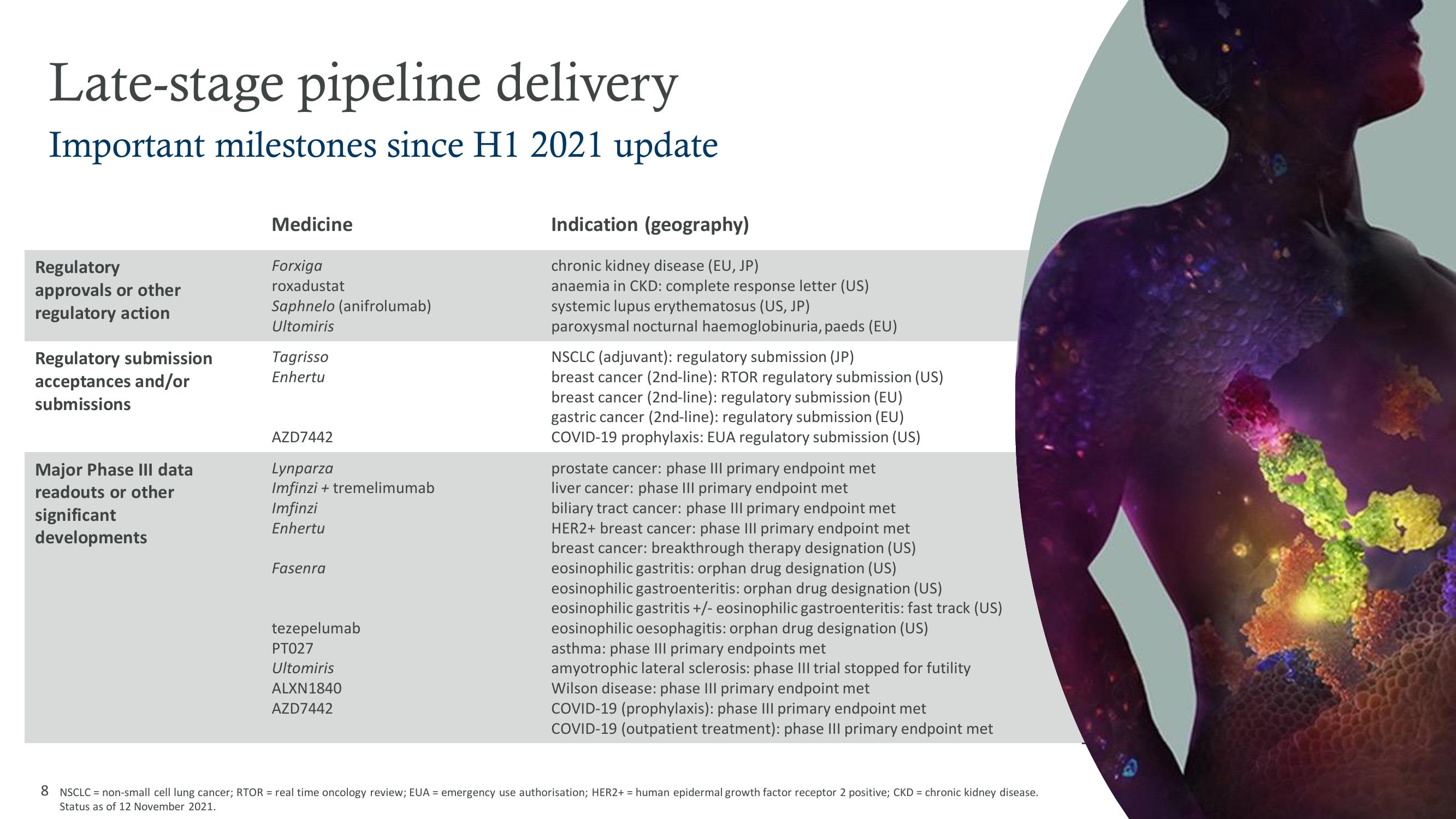
Late-stage pipeline delivery
Important milestones since H1 2021 update
Regulatory
approvals or other
regulatory action
Regulatory submission
acceptances and/or
submissions
Major Phase III data
readouts or other
significant
developments
Medicine
Forxiga
roxadustat
Saphnelo (anifrolumab)
Ultomiris
Tagrisso
Enhertu
AZD7442
Lynparza
Imfinzi + tremelimumab
Imfinzi
Enhertu
Fasenra
tezepelumab
PT027
Ultomiris
ALXN1840
AZD7442
Indication (geography)
chronic kidney disease (EU, JP)
anaemia in CKD: complete response letter (US)
systemic lupus erythematosus (US, JP)
paroxysmal nocturnal haemoglobinuria, paeds (EU)
NSCLC (adjuvant): regulatory submission (JP)
breast cancer (2nd-line): RTOR regulatory submission (US)
breast cancer (2nd-line): regulatory submission (EU)
gastric cancer (2nd-line): regulatory submission (EU)
COVID-19 prophylaxis: EUA regulatory submission (US)
prostate cancer: phase III primary endpoint met
liver cancer: phase III primary endpoint met
biliary tract cancer: phase III primary endpoint met
HER2+ breast cancer: phase III primary endpoint met
breast cancer: breakthrough therapy designation (US)
eosinophilic gastritis: orphan drug designation (US)
eosinophilic gastroenteritis: orphan drug designation (US)
eosinophilic gastritis +/- eosinophilic gastroenteritis: fast track (US)
eosinophilic oesophagitis: orphan drug designation (US)
asthma: phase III primary endpoints met
amyotrophic lateral sclerosis: phase III trial stopped for futility
Wilson disease: phase III primary endpoint met
COVID-19 (prophylaxis): phase III primary endpoint met
COVID-19 (outpatient treatment): phase III primary endpoint met
8 NSCLC = non-small cell lung cancer; RTOR = real time oncology review; EUA = emergency use authorisation; HER2+ = human epidermal growth factor receptor 2 positive; CKD = chronic kidney disease.
Status as of 12 November 2021.View entire presentation