AstraZeneca Investor Day Presentation Deck
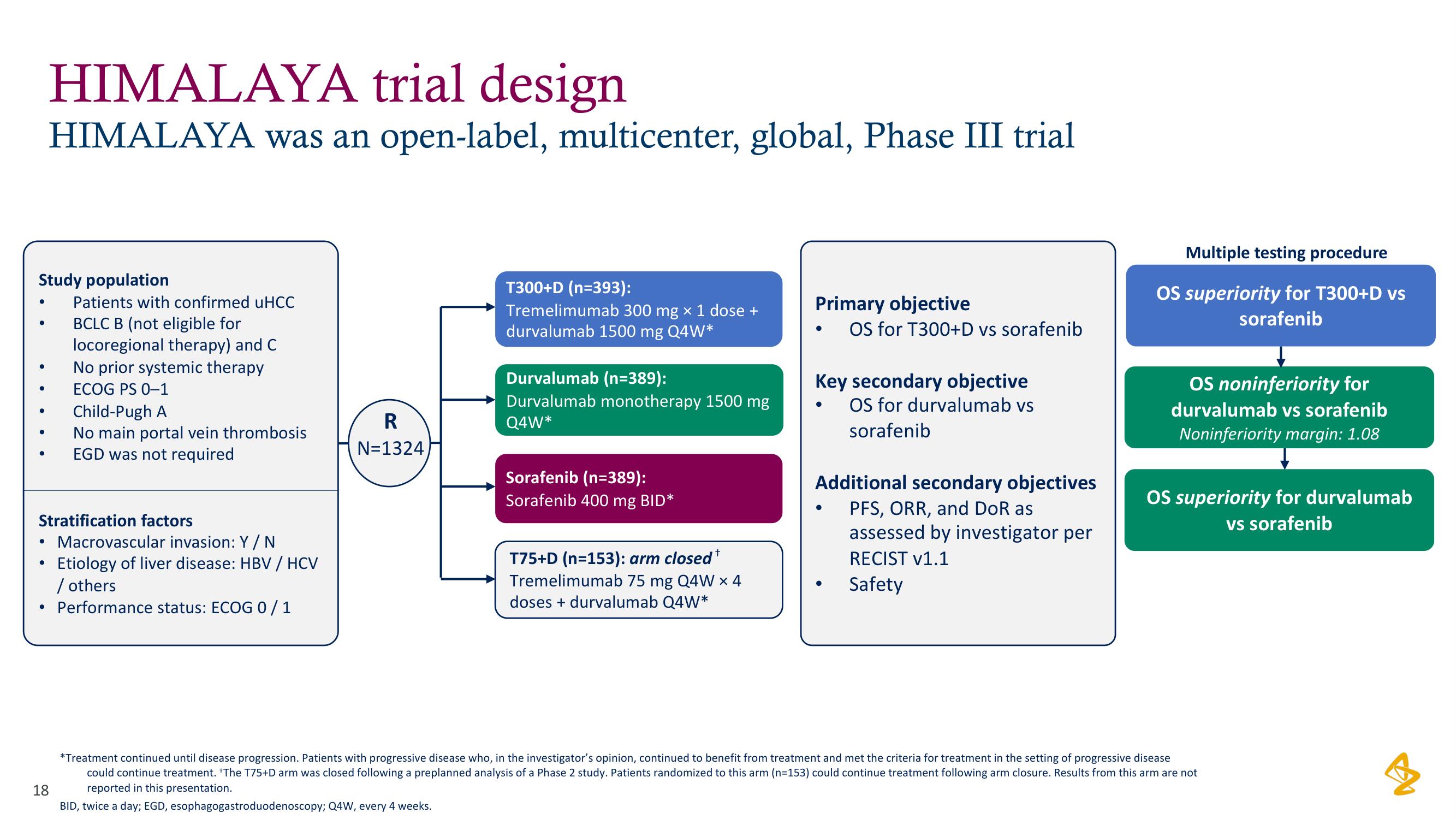
Study population
●
●
●
●
● ECOG PS 0-1
●
●
HIMALAYA trial design
HIMALAYA was an open-label, multicenter, global, Phase III trial
●
●
Patients with confirmed uHCC
BCLC B (not eligible for
locoregional therapy) and C
No prior systemic therapy
Stratification factors
Macrovascular invasion: Y / N
Etiology of liver disease: HBV / HCV
/ others
• Performance status: ECOG 0/1
18
Child-Pugh A
No main portal vein thrombosis
EGD was not required
R
N=1324
T300+D (n=393):
Tremelimumab 300 mg x 1 dose +
durvalumab 1500 mg Q4W*
Durvalumab (n=389):
Durvalumab monotherapy 1500 mg
Q4W*
Sorafenib (n=389):
Sorafenib 400 mg BID*
+
T75+D (n=153): arm closed
Tremelimumab 75 mg Q4W x 4
doses + durvalumab Q4W*
Primary objective
OS for T300+D vs sorafenib
Key secondary objective
OS for durvalumab vs
sorafenib
Additional secondary objectives
PFS, ORR, and DoR as
assessed by investigator per
RECIST v1.1
Safety
Multiple testing procedure
OS superiority for T300+D vs
sorafenib
OS noninferiority for
durvalumab vs sorafenib
Noninferiority margin: 1.08
OS superiority for durvalumab
vs sorafenib
*Treatment continued until disease progression. Patients with progressive disease who, in the investigator's opinion, continued to benefit from treatment and met the criteria for treatment in the setting of progressive disease
could continue treatment. The T75+D arm was closed following a preplanned analysis of a Phase 2 study. Patients randomized to this arm (n=153) could continue treatment following arm closure. Results from this arm are not
reported in this presentation.
BID, twice a day; EGD, esophagogastroduodenoscopy; Q4W, every 4 weeks.
3View entire presentation