AstraZeneca Investor Day Presentation Deck
100%
Percentage of Patients
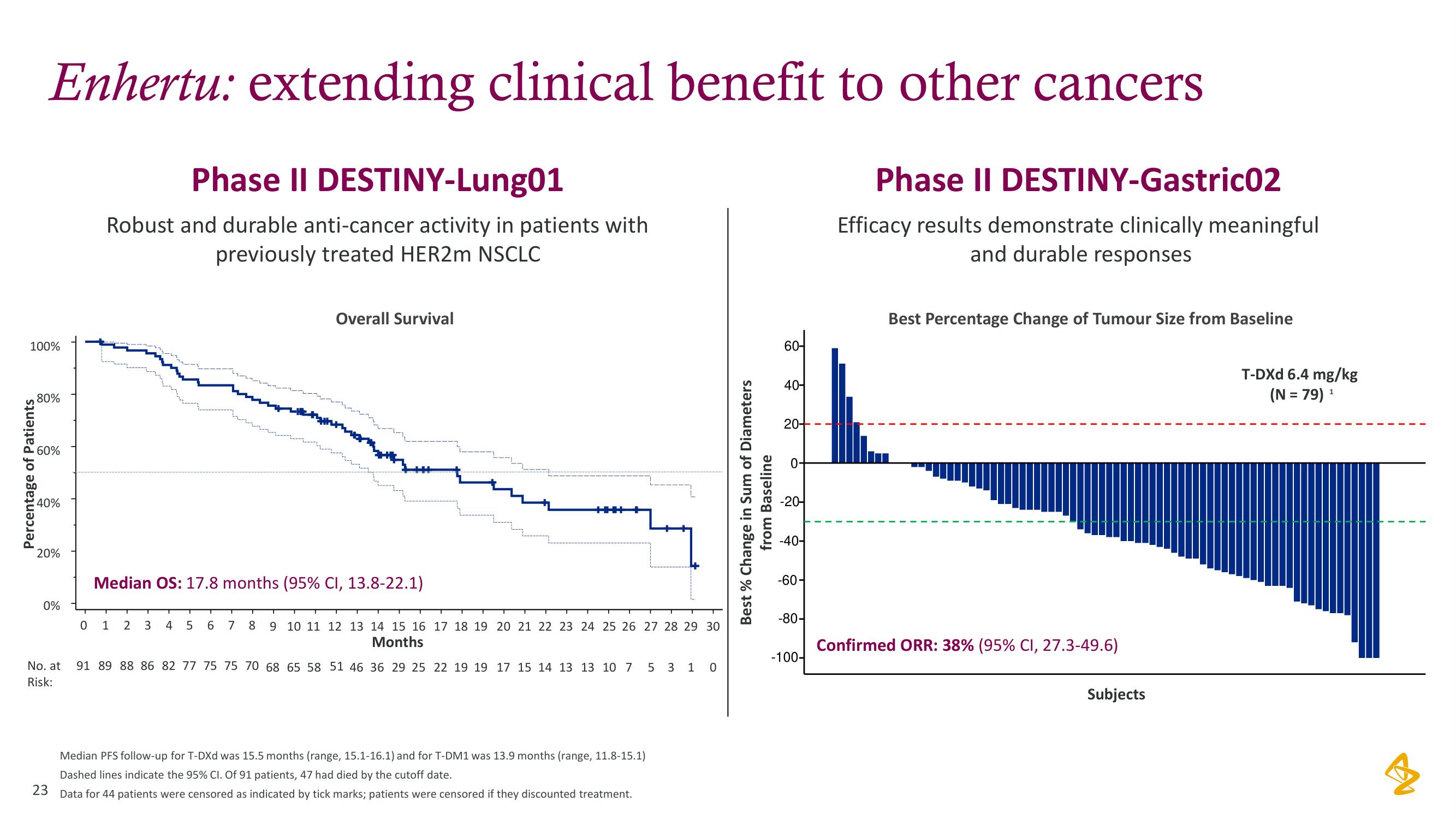
Enhertu: extending clinical benefit to other cancers
80%
60%
40%
20%
0%
No. at
Risk:
23
Phase II DESTINY-Lung01
Robust and durable anti-cancer activity in patients with
previously treated HER2m NSCLC
Overall Survival
++
Median OS: 17.8 months (95% CI, 13.8-22.1)
0
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30
Months
91 89 88 86 82 77 75 75 70 68 65 58 51 46 36 29 25 22 19 19 17 15 14 13 13 10 7 5
Median PFS follow-up for T-DXd was 15.5 months (range, 15.1-16.1) and for T-DM1 was 13.9 months (range, 11.8-15.1)
Dashed lines indicate the 95% CI. Of 91 patients, 47 had died by the cutoff date.
Data for 44 patients were censored as indicated by tick marks; patients were censored if they discounted treatment.
3 1 0
Best % Change in Sum of Diameters
from Baseline
60-
40-
20+
-20-
-40-
-60-
-80-
-100-
Phase II DESTINY-Gastric02
Efficacy results demonstrate clinically meaningful
and durable responses
Best Percentage Change of Tumour Size from Baseline
Confirmed ORR: 38% (95% CI, 27.3-49.6)
Subjects
T-DXd 6.4 mg/kg
(N = 79) ¹
3View entire presentation