AstraZeneca Investor Day Presentation Deck
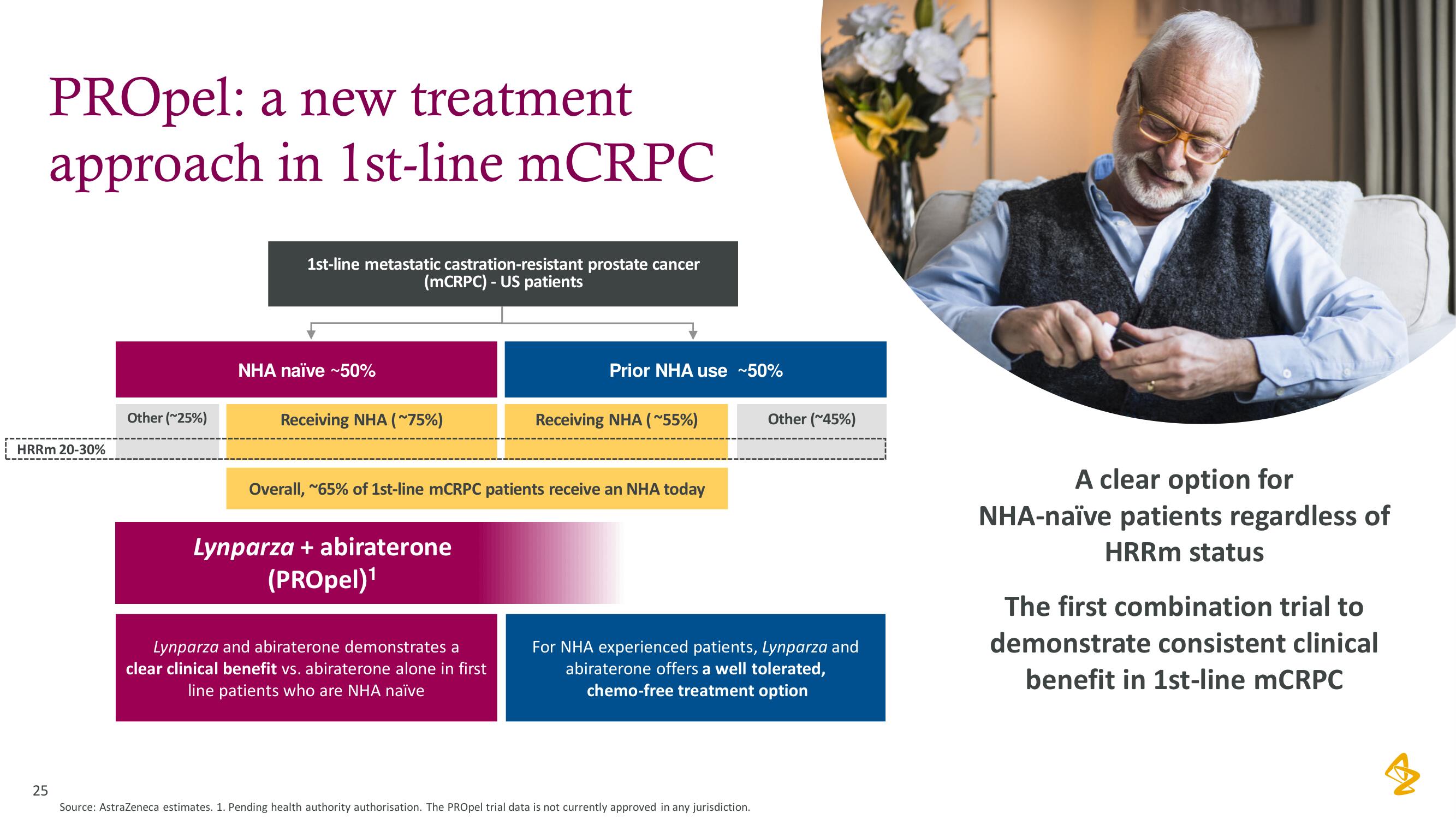
PROpel: a new treatment
approach in 1st-line mCRPC
HRRm 20-30%
25
Other (~25%)
1st-line metastatic castration-resistant prostate cancer
(mCRPC) - US patients
NHA naive ~50%
Receiving NHA (~75%)
Lynparza + abiraterone
(PROpel)¹
Prior NHA use ~50%
Overall, ~65% of 1st-line mCRPC patients receive an NHA today
Lynparza and abiraterone demonstrates a
clear clinical benefit vs. abiraterone alone in first
line patients who are NHA naïve
Receiving NHA (~55%)
Other (~45%)
For NHA experienced patients, Lynparza and
abiraterone offers a well tolerated,
chemo-free treatment option
Source: AstraZeneca estimates. 1. Pending health authority authorisation. The PROpel trial data is not currently approved in any jurisdiction.
A clear option for
NHA-naïve patients regardless of
HRRm status
The first combination trial to
demonstrate consistent clinical
benefit in 1st-line mCRPC
3View entire presentation