AstraZeneca Investor Day Presentation Deck
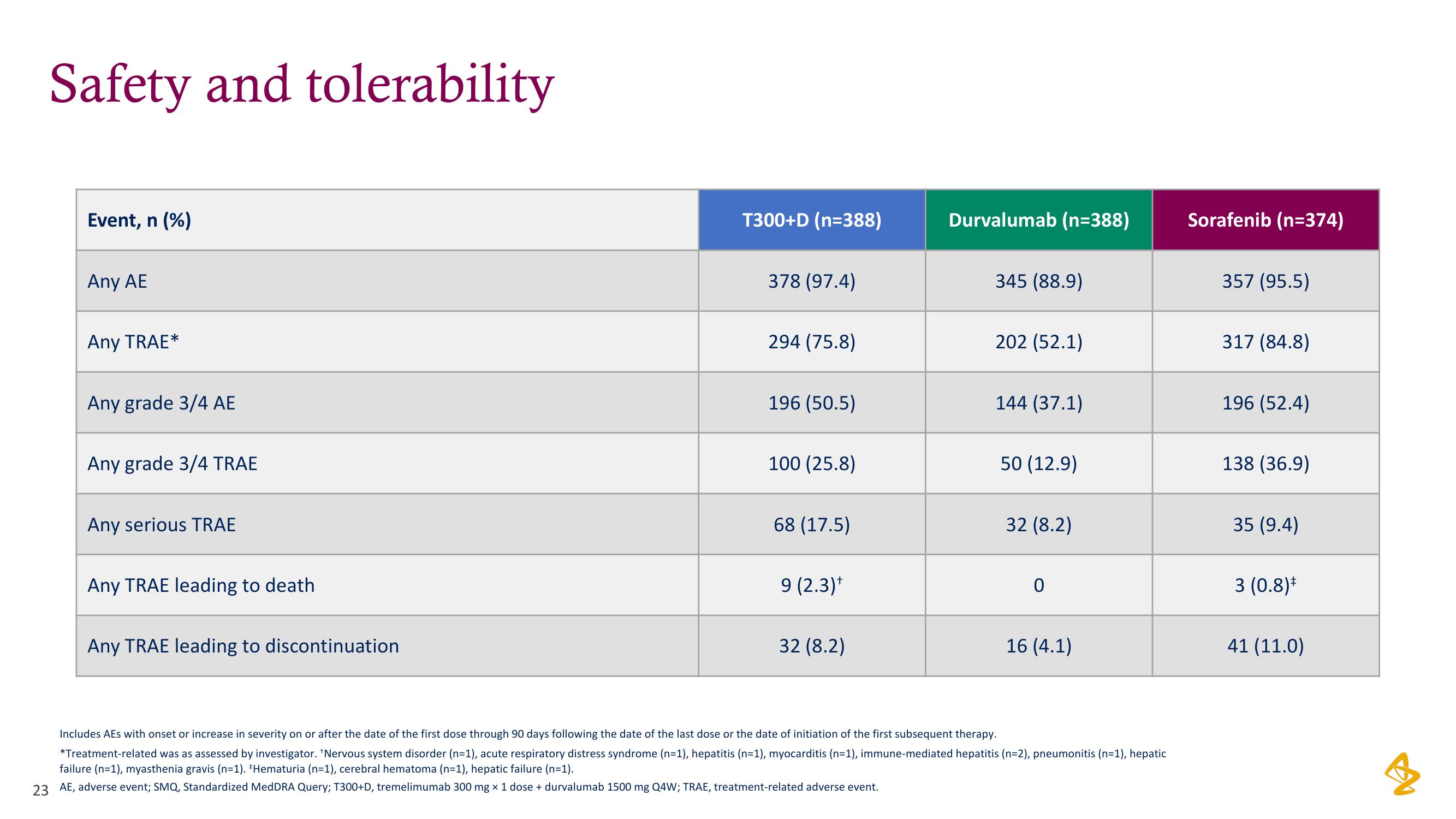
Safety and tolerability
Event, n (%)
Any AE
Any TRAE*
Any grade 3/4 AE
Any grade 3/4 TRAE
Any serious TRAE
Any TRAE leading to death
Any TRAE leading to discontinuation
T300+D (n=388)
378 (97.4)
294 (75.8)
196 (50.5)
100 (25.8)
68 (17.5)
9 (2.3)*
32 (8.2)
Durvalumab (n=388)
345 (88.9)
202 (52.1)
144 (37.1)
50 (12.9)
32 (8.2)
0
16 (4.1)
Includes AEs with onset or increase in severity on or after the date of the first dose through 90 days following the date of the last dose or the date of initiation of the first subsequent therapy.
*Treatment-related was as assessed by investigator. *Nervous system disorder (n=1), acute respiratory distress syndrome (n=1), hepatitis (n=1), myocarditis (n=1), immune-mediated hepatitis (n=2), pneumonitis (n=1), hepatic
failure (n=1), myasthenia gravis (n=1). #Hematuria (n=1), cerebral hematoma (n=1), hepatic failure (n=1).
23 AE, adverse event; SMQ, Standardized MedDRA Query; T300+D, tremelimumab 300 mg x 1 dose + durvalumab 1500 mg Q4W; TRAE, treatment-related adverse event.
Sorafenib (n=374)
357 (95.5)
317 (84.8)
196 (52.4)
138 (36.9)
35 (9.4)
3 (0.8)*
41 (11.0)
BView entire presentation