AstraZeneca Investor Day Presentation Deck
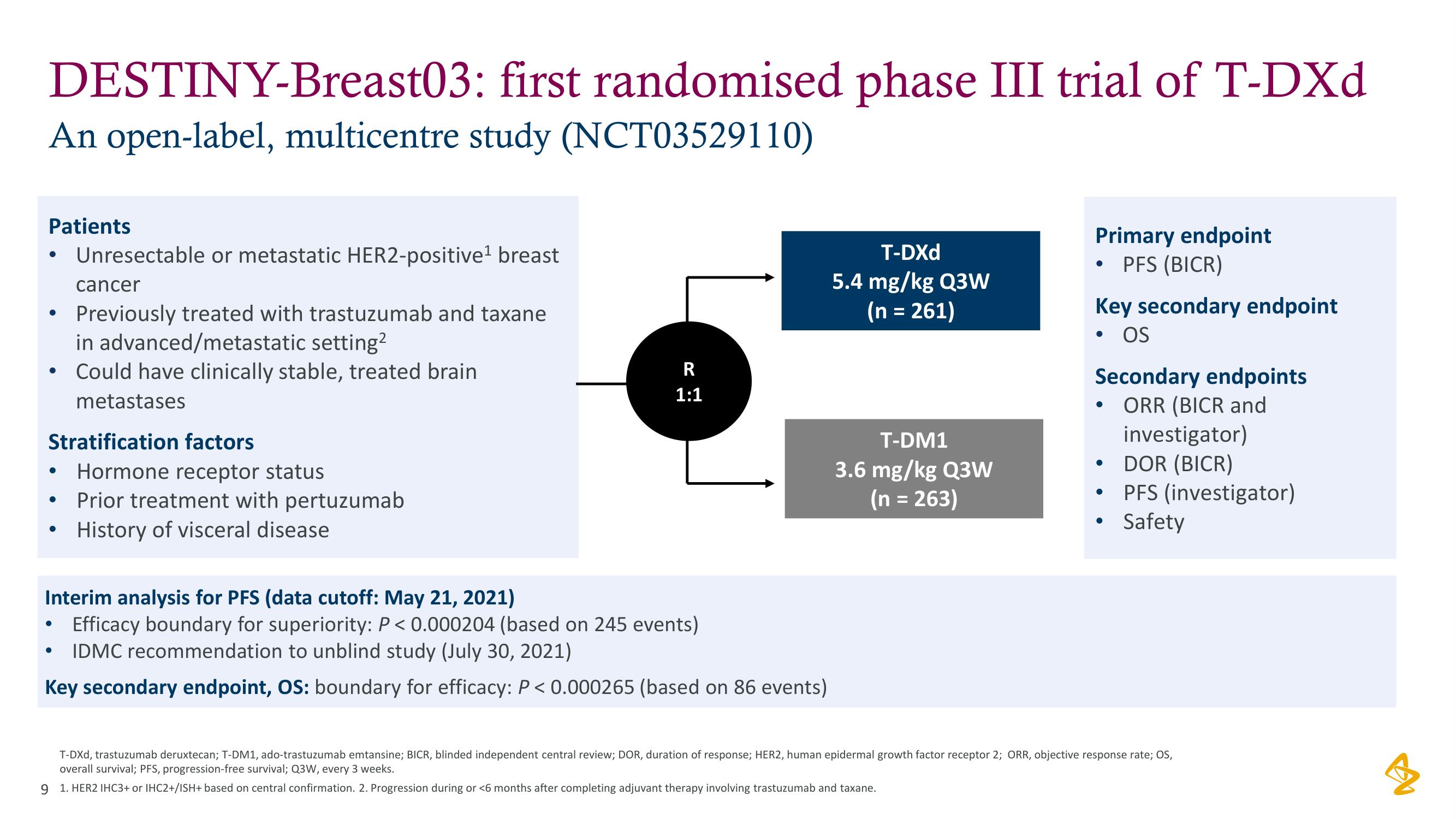
DESTINY-Breast03:
An open-label, multicentre study (NCT03529110)
Patients
●
cancer
Previously treated with trastuzumab and taxane
in advanced/metastatic setting²
• Could have clinically stable, treated brain
metastases
Stratification factors
●
first randomised phase III trial of T-DXd
Unresectable or metastatic HER2-positive¹ breast
●
Hormone receptor status
Prior treatment with pertuzumab
History of visceral disease
R
1:1
Interim analysis for PFS (data cutoff: May 21, 2021)
• Efficacy boundary for superiority: P < 0.000204 (based on 245 events)
IDMC recommendation to unblind study (July 30, 2021)
Key secondary endpoint, OS: boundary for efficacy: P < 0.000265 (based on 86 events)
T-DXd
5.4 mg/kg Q3W
(n = 261)
T-DM1
3.6 mg/kg Q3W
(n = 263)
Primary endpoint
PFS (BICR)
Key secondary endpoint
OS
Secondary endpoints
ORR (BICR and
investigator)
●
DOR (BICR)
• PFS (investigator)
Safety
●
●
T-DXd, trastuzumab deruxtecan; T-DM1, ado-trastuzumab emtansine; BICR, blinded independent central review; DOR, duration of response; HER2, human epidermal growth factor receptor 2; ORR, objective response rate; OS,
overall survival; PFS, progression-free survival; Q3W, every 3 weeks.
9 1. HER2 IHC3+ or IHC2+/ISH+ based on central confirmation. 2. Progression during or <6 months after completing adjuvant therapy involving trastuzumab and taxane.
3View entire presentation