AstraZeneca Investor Day Presentation Deck
15
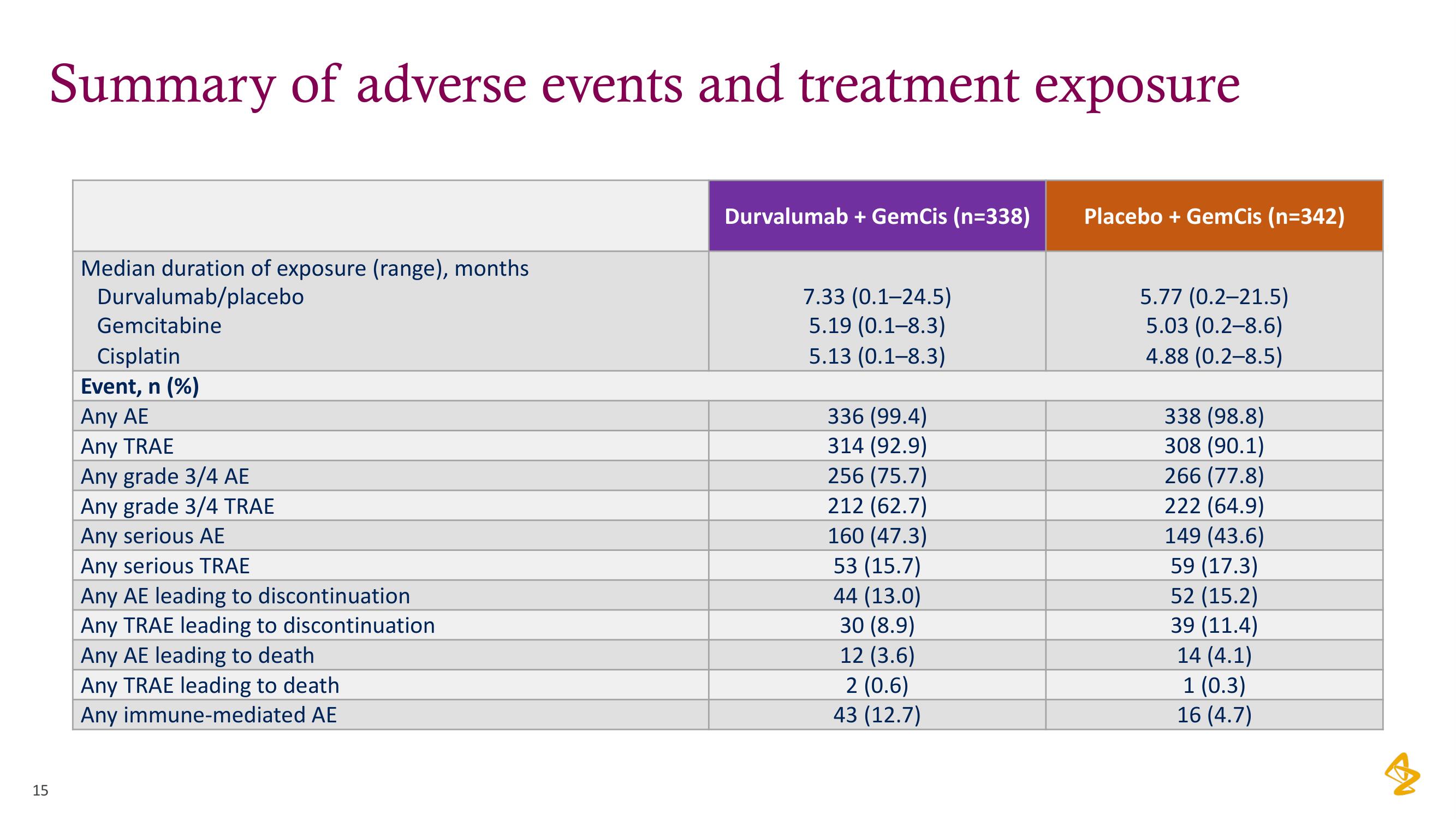
Summary of adverse events and treatment exposure
Median duration of exposure (range), months
Durvalumab/placebo
Gemcitabine
Cisplatin
Event, n (%)
Any AE
Any TRAE
Any grade 3/4 AE
Any grade 3/4 TRAE
Any serious AE
Any serious TRAE
Any AE leading to discontinuation
Any TRAE leading to discontinuation
Any AE leading to death
Any TRAE leading to death
Any immune-mediated AE
Durvalumab + GemCis (n=338)
7.33 (0.1-24.5)
5.19 (0.1-8.3)
5.13 (0.1-8.3)
336 (99.4)
314 (92.9)
256 (75.7)
212 (62.7)
160 (47.3)
53 (15.7)
44 (13.0)
30 (8.9)
12 (3.6)
2 (0.6)
43 (12.7)
Placebo + GemCis (n=342)
5.77 (0.2-21.5)
5.03 (0.2-8.6)
4.88 (0.2-8.5)
338 (98.8)
308 (90.1)
266 (77.8)
222 (64.9)
149 (43.6)
59 (17.3)
52
(15.2)
39 (11.4)
14 (4.1)
1 (0.3)
16 (4.7)View entire presentation