AstraZeneca Investor Day Presentation Deck
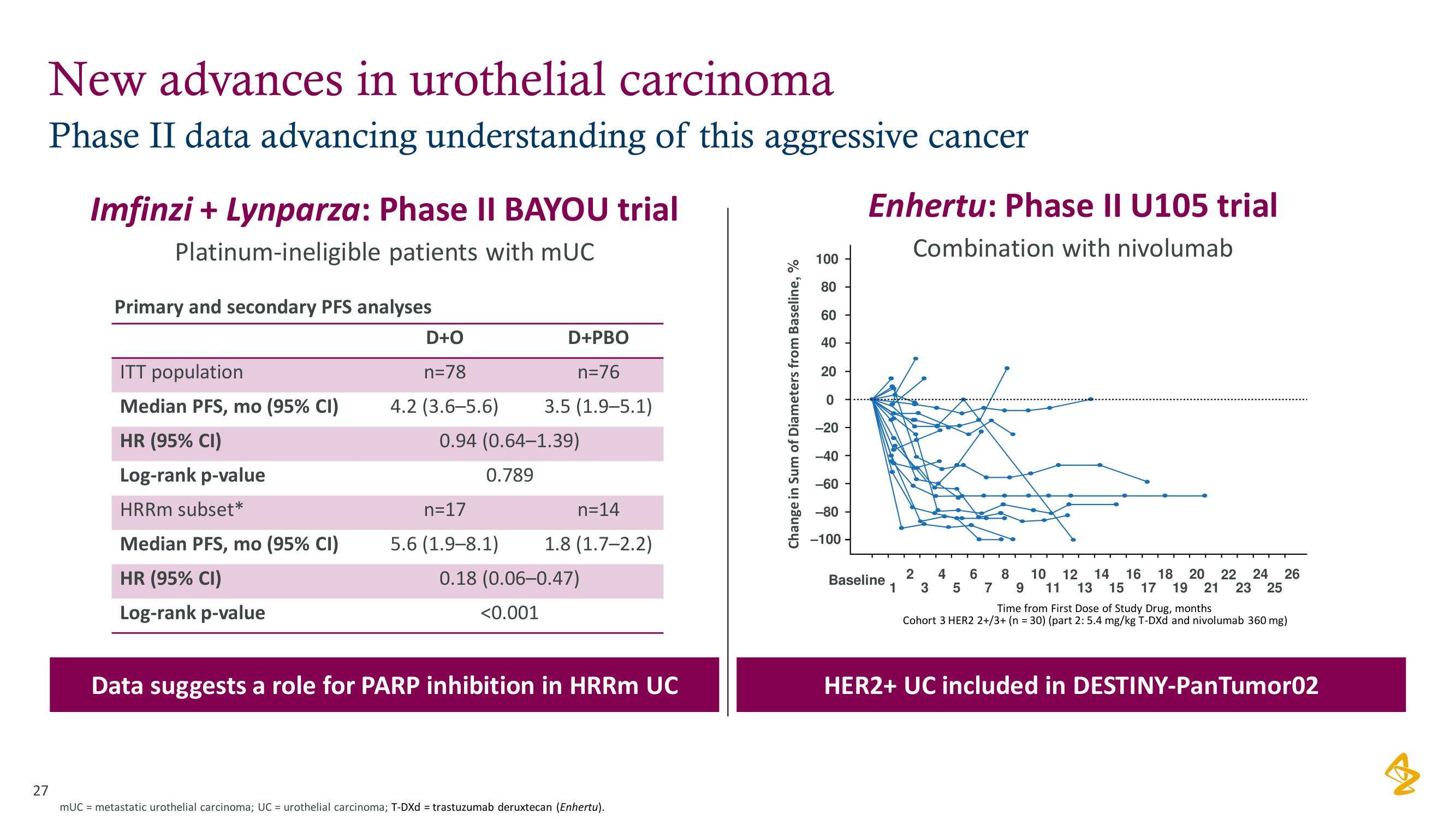
New advances in urothelial carcinoma
Phase II data advancing understanding of this aggressive cancer
27
Imfinzi + Lynparza: Phase II BAYOU trial
Platinum-ineligible patients with mUC
Primary and secondary PFS analyses
ITT population
Median PFS, mo (95% CI)
HR (95% CI)
Log-rank p-value
HRRm subset*
Median PFS, mo (95% CI)
HR (95% CI)
Log-rank p-value
D+O
n=78
4.2 (3.6-5.6)
D+PBO
n=76
3.5 (1.9-5.1)
0.94 (0.64-1.39)
0.789
n=17
5.6 (1.9-8.1)
n=14
1.8 (1.7-2.2)
0.18 (0.06-0.47)
<0.001
Data suggests a role for PARP inhibition in HRRm UC
mUC = metastatic urothelial carcinoma; UC = urothelial carcinoma; T-DXd = trastuzumab deruxtecan (Enhertu).
Change in Sum of Diameters from Baseline, %
100
80
60
40
20
0
-20
-40
-60
-80
-100-
Enhertu: Phase II U105 trial
Combination with nivolumab
Baseline
1
2
3
4
6
8 10 12 14 16 18 20 22 24 26
5 7 9 11 13 15 17 19 21 23 25
Time from First Dose of Study Drug, months
Cohort 3 HER2 2+/3+ (n = 30) (part 2: 5.4 mg/kg T-DXd and nivolumab 360 mg)
HER2+ UC included in DESTINY-PanTumor02View entire presentation