AstraZeneca Investor Day Presentation Deck
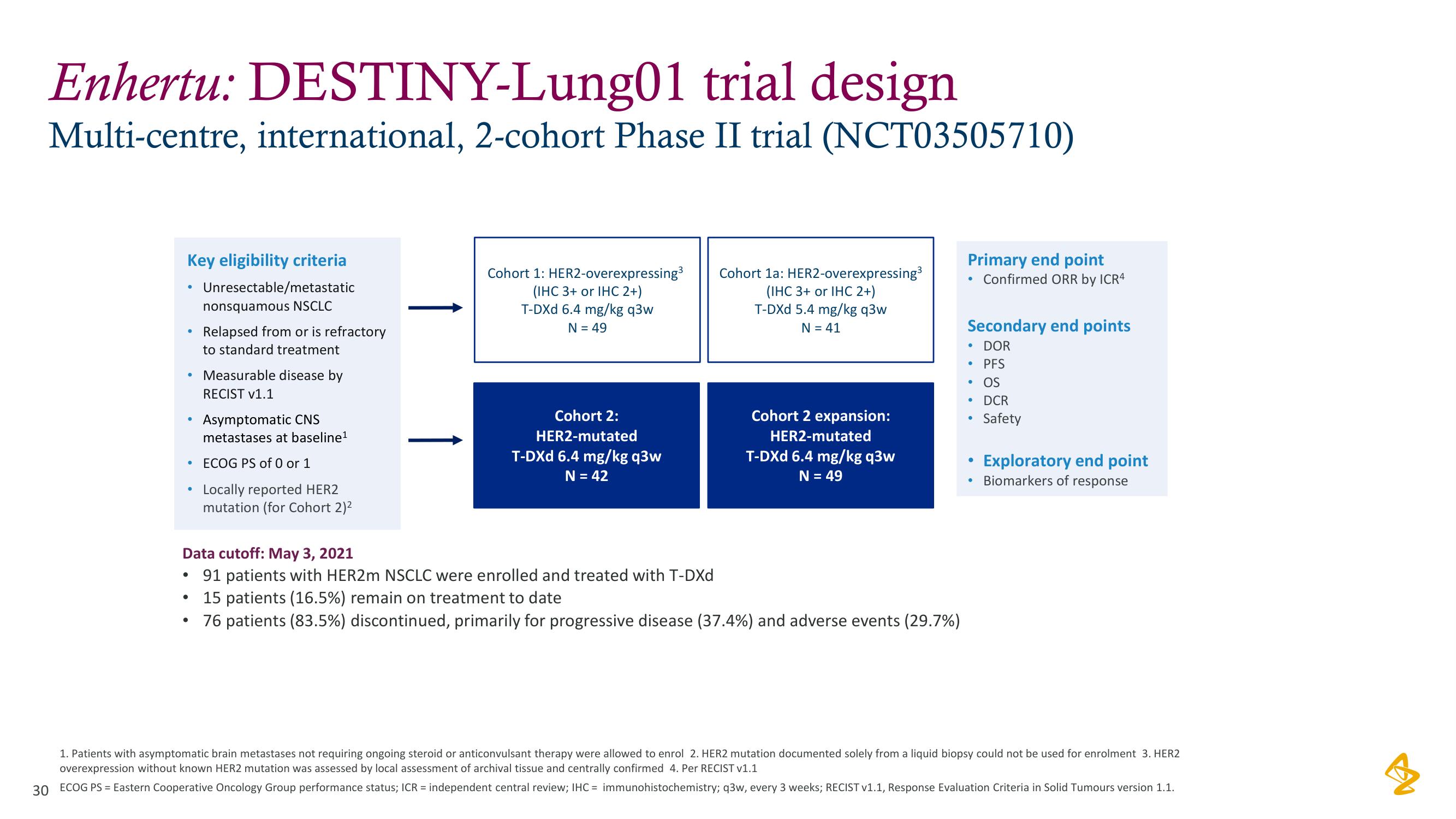
Enhertu: DESTINY-Lung01 trial design
Multi-centre, international, 2-cohort Phase II trial (NCT03505710)
Key eligibility criteria
Unresectable/metastatic
●
●
●
●
●
●
nonsquamous NSCLC
Relapsed from or is refractory
to standard treatment
●
Measurable disease by
RECIST v1.1
Asymptomatic CNS
metastases at baseline¹
ECOG PS of 0 or 1
Locally reported HER2
mutation (for Cohort 2)²
Cohort 1: HER2-overexpressing³
(IHC 3+ or IHC 2+)
T-DXd 6.4 mg/kg q3w
N = 49
Cohort 2:
HER2-mutated
T-DXd 6.4 mg/kg q3w
N = 42
Cohort 1a: HER2-overexpressing³
(IHC 3+ or IHC 2+)
T-DXd 5.4 mg/kg q3w
N = 41
Cohort 2 expansion:
HER2-mutated
T-DXd 6.4 mg/kg q3w
N = 49
Data cutoff: May 3, 2021
• 91 patients with HER2m NSCLC were enrolled and treated with T-DXd
15 patients (16.5%) remain on treatment to date
76 patients (83.5%) discontinued, primarily for progressive disease (37.4%) and adverse events (29.7%)
Primary end point
Confirmed ORR by ICR4
Secondary end points
• DOR
• PFS
• OS
• DCR
●
Safety
Exploratory end point
Biomarkers of response
1. Patients with asymptomatic brain metastases not requiring ongoing steroid or anticonvulsant therapy were allowed to enrol 2. HER2 mutation documented solely from a liquid biopsy could not be used for enrolment 3. HER2
overexpression without known HER2 mutation was assessed by local assessment of archival tissue and centrally confirmed 4. Per RECIST v1.1
30 ECOG PS= Eastern Cooperative Oncology Group performance status; ICR = independent central review; IHC = immunohistochemistry; q3w, every 3 weeks; RECIST v1.1, Response Evaluation Criteria in Solid Tumours version 1.1.
3View entire presentation