AstraZeneca Investor Day Presentation Deck
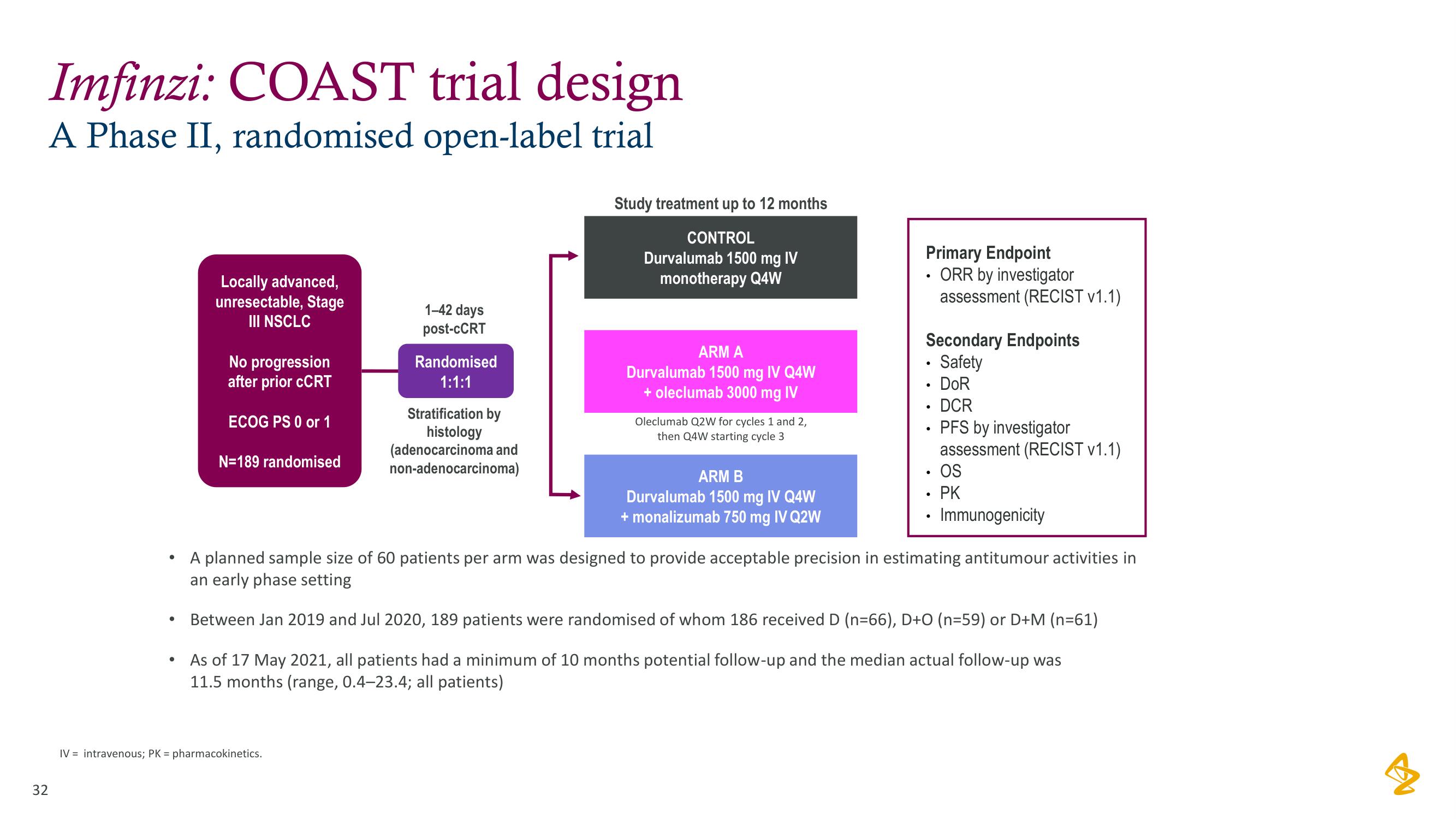
Imfinzi: COAST trial design
A Phase II, randomised open-label trial
32
●
●
●
Locally advanced,
unresectable, Stage
III NSCLC
No progression
after prior CCRT
ECOG PS 0 or 1
N=189 randomised
1-42 days
post-cCRT
IV = intravenous; PK = pharmacokinetics.
Randomised
1:1:1
Stratification by
histology
(adenocarcinoma and
non-adenocarcinoma)
Study treatment up to 12 months
CONTROL
Durvalumab 1500 mg IV
monotherapy Q4W
ARM A
Durvalumab 1500 mg IV Q4W
+ oleclumab 3000 mg IV
Oleclumab Q2W for cycles 1 and 2,
then Q4W starting cycle 3
ARM B
Durvalumab 1500 mg IV Q4W
+ monalizumab 750 mg IV Q2W
Primary Endpoint
ORR by investigator
assessment (RECIST v1.1)
Secondary Endpoints
• Safety
• DOR
• DCR
• PFS by investigator
assessment (RECIST v1.1)
• OS
.
• PK
Immunogenicity
A planned sample size of 60 patients per arm was designed to provide acceptable precision in estimating antitumour activities in
an early phase setting
Between Jan 2019 and Jul 2020, 189 patients were randomised of whom 186 received D (n=66), D+O (n=59) or D+M (n=61)
As of 17 May 2021, all patients had a minimum of 10 months potential follow-up and the median actual follow-up was
11.5 months (range, 0.4-23.4; all patients)
●
BView entire presentation