AstraZeneca Investor Day Presentation Deck
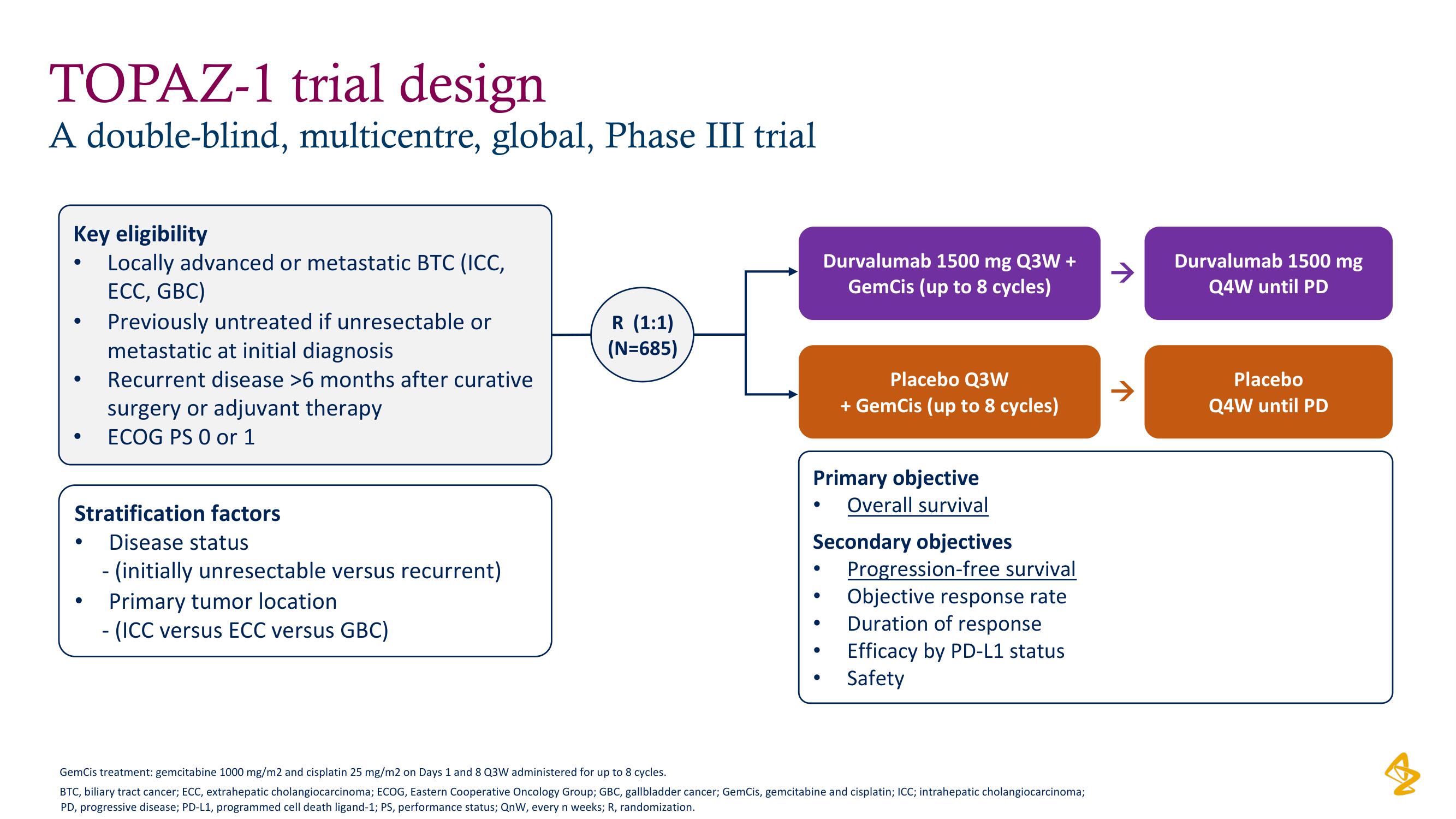
TOPAZ-1 trial design
A double-blind, multicentre, global, Phase III trial
Key eligibility
Locally advanced or metastatic BTC (ICC,
ECC, GBC)
●
●
Previously untreated if unresectable or
metastatic at initial diagnosis
Recurrent disease >6 months after curative
surgery or adjuvant therapy
ECOG PS 0 or 1
Stratification factors
Disease status
- (initially unresectable versus recurrent)
Primary tumor location
- (ICC versus ECC versus GBC)
R (1:1)
(N=685)
Durvalumab 1500 mg Q3W +
GemCis (up to 8 cycles)
●
Placebo Q3W
+ GemCis (up to 8 cycles)
Primary objective
Overall survival
Secondary objectives
Progression-free survival
Objective response rate
Duration of response
Efficacy by PD-L1 status
Safety
GemCis treatment: gemcitabine 1000 mg/m2 and cisplatin 25 mg/m2 on Days 1 and 8 Q3W administered for up to 8 cycles.
BTC, biliary tract cancer; ECC, extrahepatic cholangiocarcinoma; ECOG, Eastern Cooperative Oncology Group; GBC, gallbladder cancer; GemCis, gemcitabine and cisplatin; ICC; intrahepatic cholangiocarcinoma;
PD, progressive disease; PD-L1, programmed cell death ligand-1; PS, performance status; QnW, every n weeks; R, randomization.
Durvalumab 1500 mg
Q4W until PD
Placebo
Q4W until PDView entire presentation