AstraZeneca Results Presentation Deck
Oncology: R&D pipeline highlights
Strong presence at ESMO and WCLC congresses
18
●
●
US FDA
BTD
received
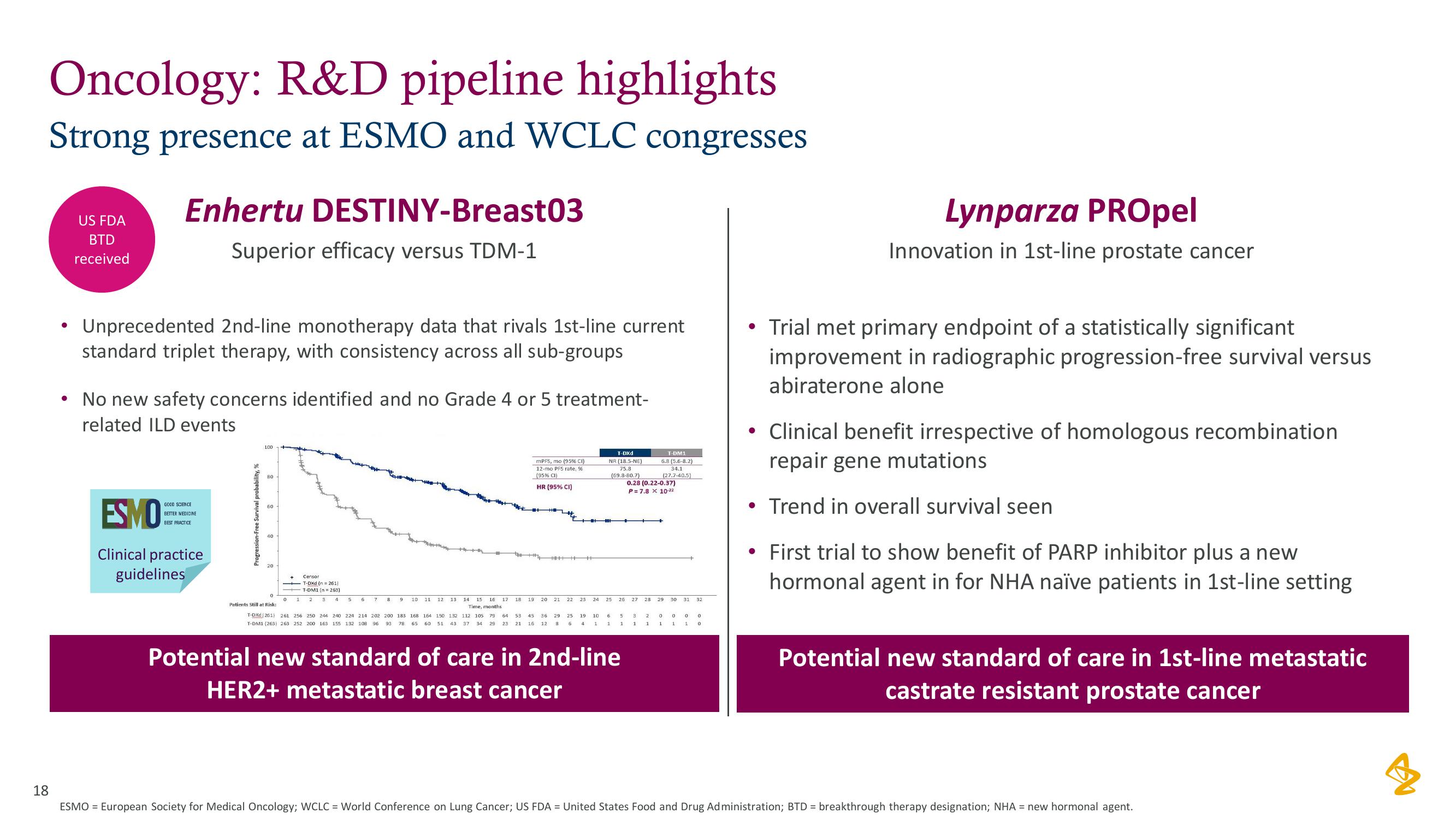
Enhertu DESTINY-Breast03
Superior efficacy versus TDM-1
Unprecedented 2nd-line monotherapy data that rivals 1st-line current
standard triplet therapy, with consistency across all sub-groups
No new safety concerns identified and no Grade 4 or 5 treatment-
related ILD events
ESMO
Clinical practice
guidelines
GOOD SCIENCE
BETTER MEDICINE
BEST PRACTICE
Progression-Free Survival pro
100
80
60
40
20
0
1
Censor
T-DXd (n = 261)
T-DM1 (n=263)
2 3. 4
5
7
8 9 10 11 12 13
14 15 16 17
Time, months
18
19
mPFS, mo (95% CI)
12-mo PFS rate, %
(95% CI)
HR (95% CI)
20 21 22 23 24 25 26
Patients Still at Risk:
T-DXd 251) 261 256 250 244 240 224 214 202 200 183 168 164 150 132
112 105 79 64
T-DM1 (263) 263 252 200 163 155 132 108 96 93 78 65 60 51 43 37 34 29 23 21 16 12 B
T-DXd
NR (18.5-NE)
75.8
(69.8-80.7)
53 45 36 29 25 19 10
6
4
1
6
1
5
1
Potential new standard of care in 2nd-line
HER2+ metastatic breast cancer
T-DM1
6.8 (5.6-8.2)
34.1
(27.7-40.5)
0.28 (0.22-0.37)
P= 7.8 x 10-22
27 28 29
3
1 1 1
30 311 32
0 0 0
1 0
Lynparza PROpel
Innovation in 1st-line prostate cancer
• Trial met primary endpoint of a statistically significant
improvement in radiographic progression-free survival versus
abiraterone alone
Clinical benefit irrespective of homologous recombination
repair gene mutations
Trend in overall survival seen
• First trial to show benefit of PARP inhibitor plus a new
hormonal agent in for NHA naïve patients in 1st-line setting
Potential new standard of care in 1st-line metastatic
castrate resistant prostate cancer
ESMO = European Society for Medical Oncology; WCLC = World Conference on Lung Cancer; US FDA = United States Food and Drug Administration; BTD = breakthrough therapy designation; NHA = new hormonal agent.View entire presentation