AstraZeneca Investor Day Presentation Deck
11
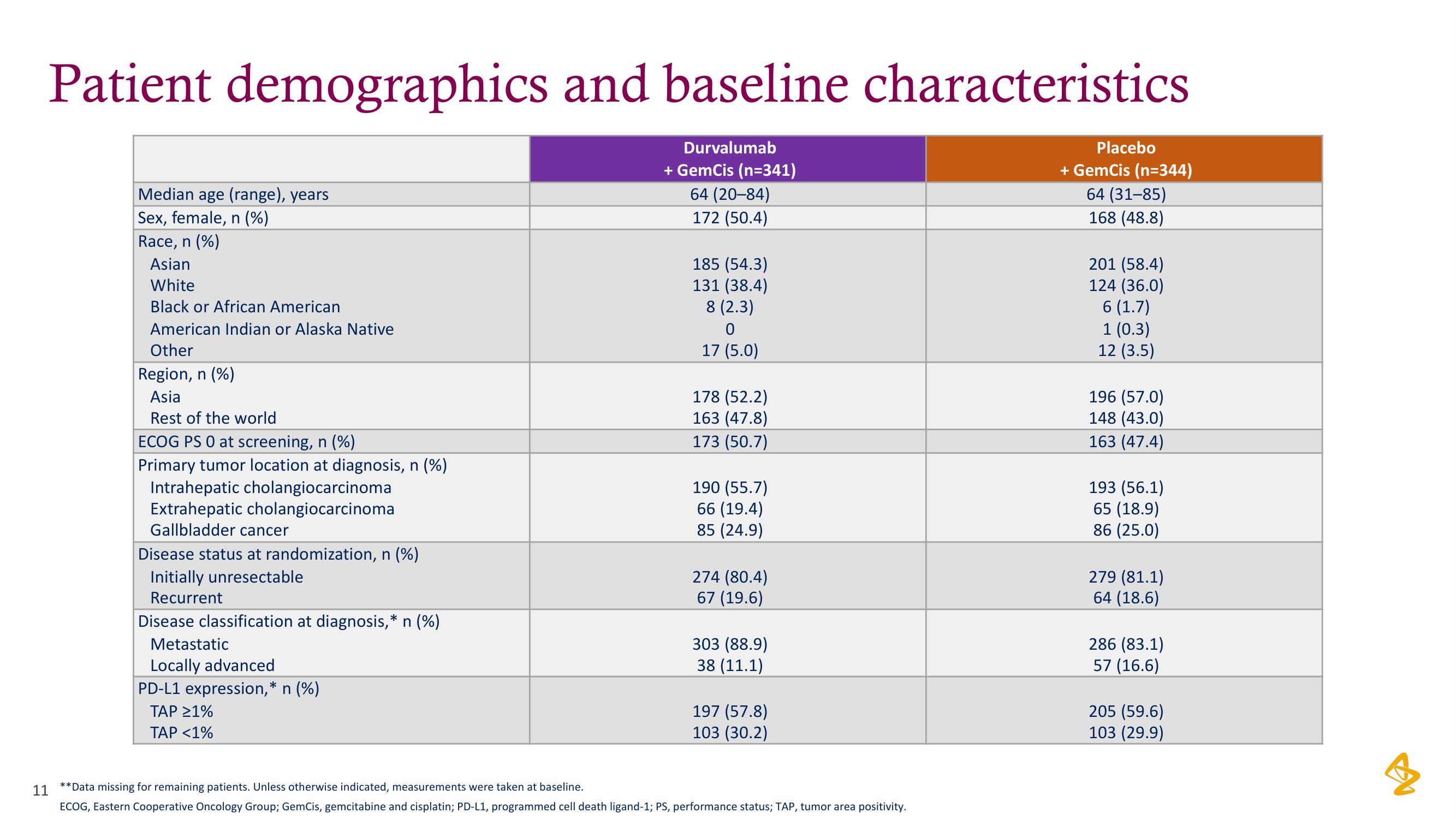
Patient demographics and baseline characteristics
Durvalumab
+ GemCis (n=341)
64 (20-84)
172 (50.4)
Median age (range), years
Sex, female, n (%)
Race, n (%)
Asian
White
Black or African American
American Indian or Alaska Native
Other
Region, n (%)
Asia
Rest of the world
ECOG PS 0 at screening, n (%)
Primary tumor location at diagnosis, n (%)
Intrahepatic cholangiocarcinoma
Extrahepatic cholangiocarcinoma
Gallbladder cancer
Disease status at randomization, n (%)
Initially unresectable
Recurrent
Disease classification at diagnosis,* n (%)
Metastatic
Locally advanced
PD-L1 expression,* n (%)
TAP ≥1%
TAP <1%
185 (54.3)
131 (38.4)
8 (2.3)
0
17 (5.0)
178 (52.2)
163 (47.8)
173 (50.7)
190 (55.7)
66 (19.4)
85 (24.9)
274 (80.4)
67 (19.6)
303 (88.9)
38 (11.1)
197 (57.8)
103 (30.2)
**Data missing for remaining patients. Unless otherwise indicated, measurements were taken at baseline.
ECOG, Eastern Cooperative Oncology Group; GemCis, gemcitabine and cisplatin; PD-L1, programmed cell death ligand-1; PS, performance status; TAP, tumor area positivity.
Placebo
+ GemCis (n=344)
64 (31-85)
168 (48.8)
201 (58.4)
124 (36.0)
6 (1.7)
1 (0.3)
12 (3.5)
196 (57.0)
148 (43.0)
163 (47.4)
193 (56.1)
65 (18.9)
86 (25.0)
279 (81.1)
64 (18.6)
286 (83.1)
57 (16.6)
205 (59.6)
103 (29.9)
BView entire presentation