AstraZeneca Results Presentation Deck
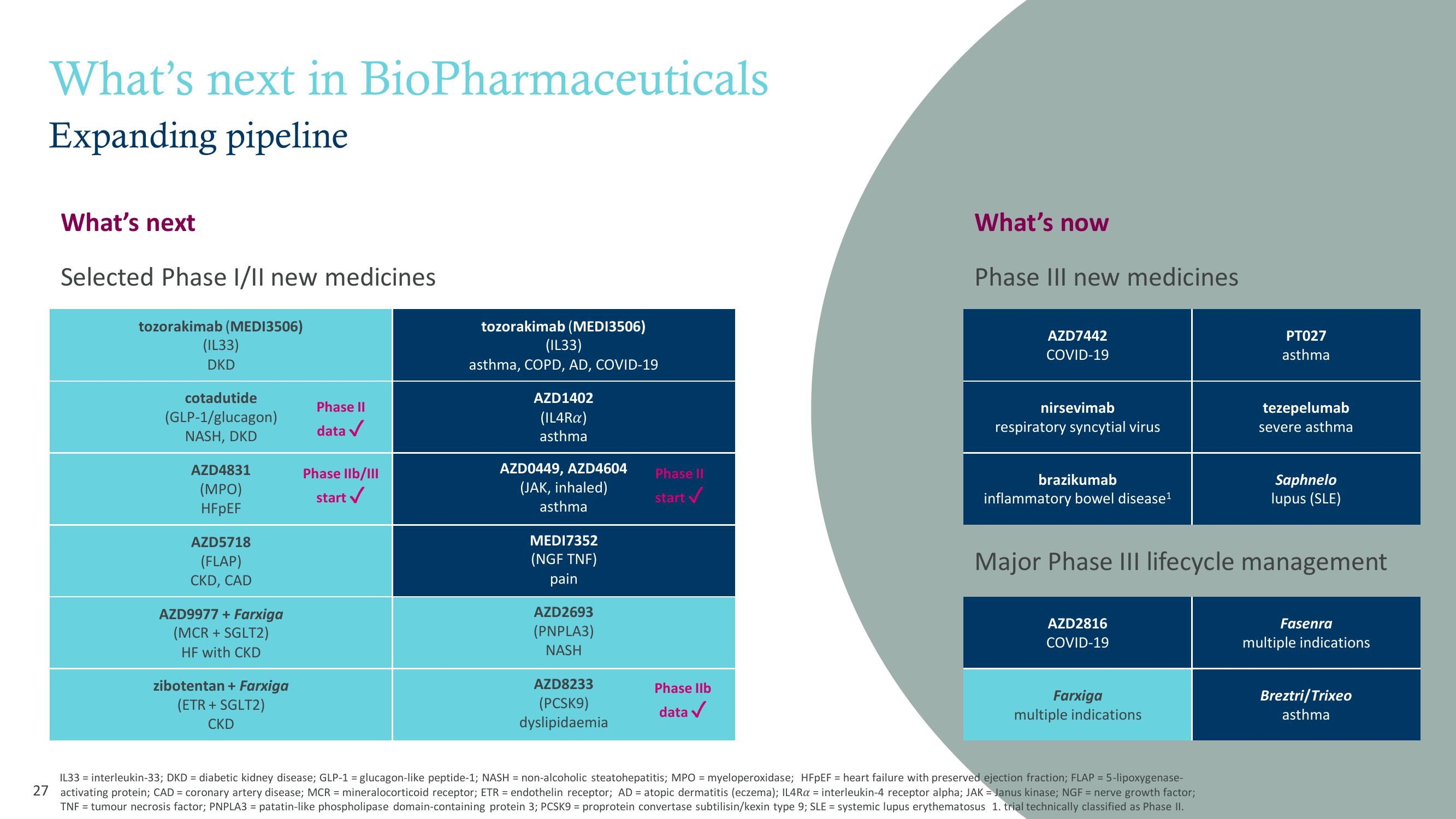
What's next in BioPharmaceuticals
Expanding pipeline
What's next
Selected Phase I/II new medicines
tozorakimab (MEDI3506)
(IL33)
DKD
cotadutide
(GLP-1/glucagon)
NASH, DKD
AZD4831
(MPO)
HFpEF
AZD5718
(FLAP)
CKD, CAD
AZD9977 + Farxiga
(MCR + SGLT2)
HF with CKD
zibotentan + Farxiga
(ETR + SGLT2)
CKD
Phase II
data ✓
Phase IIb/III
start ✓
tozorakimab (MEDI3506)
(IL33)
asthma, COPD, AD, COVID-19
AZD1402
(IL4Ra)
asthma
AZD0449, AZD4604
(JAK, inhaled)
asthma
MEDI7352
(NGF TNF)
pain
AZD2693
(PNPLA3)
NASH
AZD8233
(PCSK9)
dyslipidaemia
Phase II
start ✓
Phase llb
data ✓
What's now
Phase III new medicines
AZD7442
COVID-19
nirsevimab
respiratory syncytial virus
brazikumab
inflammatory bowel disease¹
Farxiga
multiple indications
PT027
asthma
IL33 = interleukin-33; DKD = diabetic kidney disease; GLP-1 = glucagon-like peptide-1; NASH = non-alcoholic steatohepatitis; MPO = myeloperoxidase; HFpEF = heart failure with preserved ejection fraction; FLAP = 5-lipoxygenase-
27 activating protein; CAD = coronary artery disease; MCR = mineralocorticoid receptor; ETR = endothelin receptor; AD = atopic dermatitis (eczema); IL4Rα = interleukin-4 receptor alpha; JAK = Janus kinase; NGF = nerve growth factor;
TNF = tumour necrosis factor; PNPLA3 = patatin-like phospholipase domain-containing protein 3; PCSK9 = proprotein convertase subtilisin/kexin type 9; SLE = systemic lupus erythematosus 1. trial technically classified as Phase II.
tezepelumab
severe asthma
Major Phase III lifecycle management
AZD2816
COVID-19
Saphnelo
lupus (SLE)
Fasenra
multiple indications
Breztri/Trixeo
asthmaView entire presentation