AstraZeneca Investor Day Presentation Deck
15
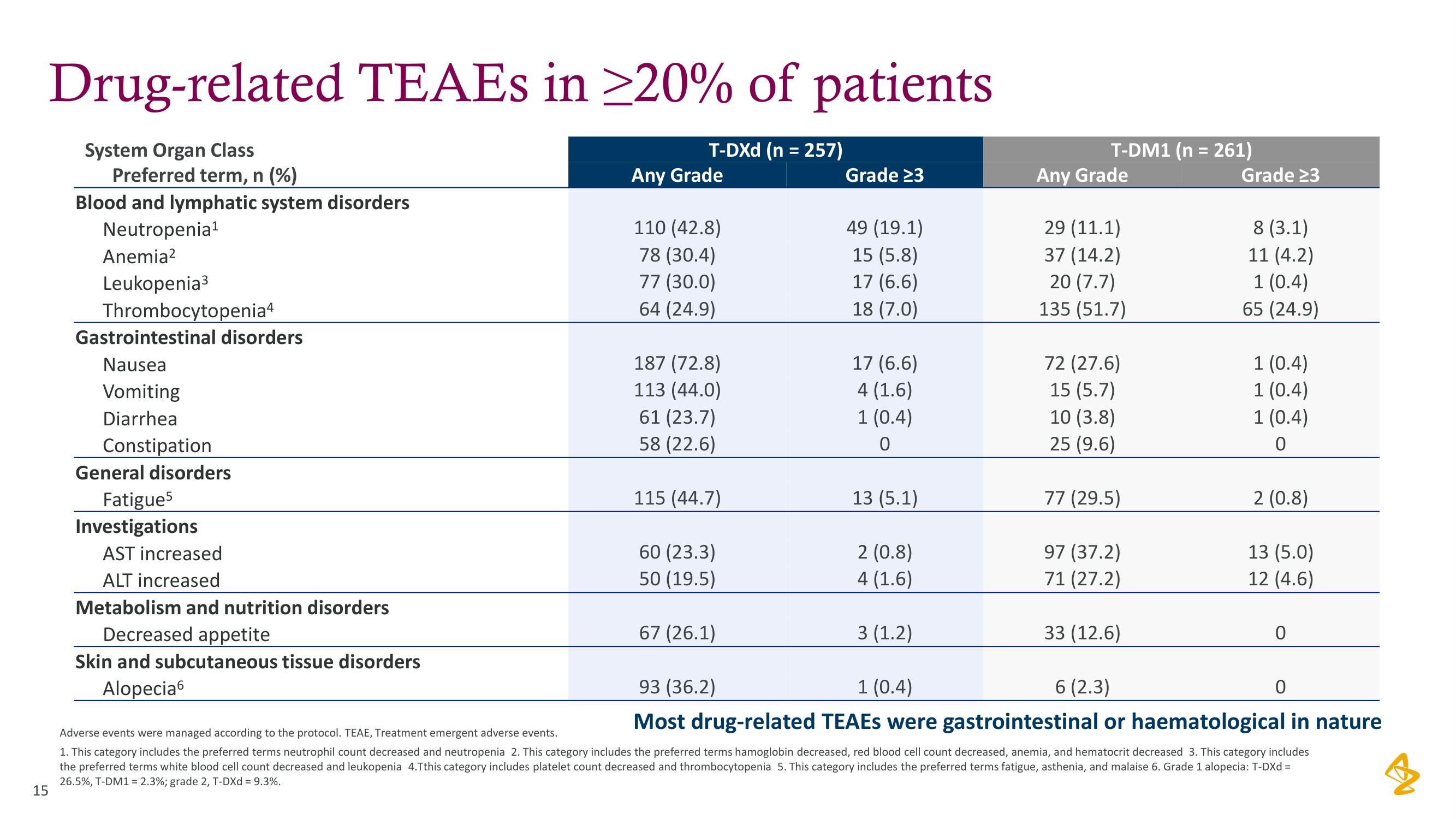
Drug-related TEAES in ≥20% of patients
T-DXd (n = 257)
System Organ Class
Preferred term, n (%)
Blood and lymphatic system disorders
Neutropenia¹
Anemia²
Leukopenia³
Thrombocytopenia4
Gastrointestinal disorders
Nausea
Vomiting
Diarrhea
Constipation
General disorders
Fatigues
Investigations
AST increased
ALT increased
Metabolism and nutrition disorders
Decreased appetite
Skin and subcutaneous tissue disorders
Alopecia
Any Grade
110 (42.8)
78 (30.4)
77 (30.0)
64 (24.9)
187 (72.8)
113 (44.0)
61 (23.7)
58 (22.6)
115 (44.7)
60 (23.3)
50 (19.5)
67 (26.1)
Grade 23
49 (19.1)
15 (5.8)
17 (6.6)
18 (7.0)
17 (6.6)
4 (1.6)
1 (0.4)
0
13 (5.1)
2 (0.8)
4 (1.6)
3 (1.2)
T-DM1 (n = 261)
Any Grade
29 (11.1)
37 (14.2)
20 (7.7)
135 (51.7)
72 (27.6)
15 (5.7)
10 (3.8)
25 (9.6)
77 (29.5)
97 (37.2)
71 (27.2)
33 (12.6)
Grade 23
8 (3.1)
11 (4.2)
1 (0.4)
65 (24.9)
1 (0.4)
1 (0.4)
1 (0.4)
0
2 (0.8)
13 (5.0)
12 (4.6)
0
93 (36.2)
1 (0.4)
6 (2.3)
0
Most drug-related TEAEs were gastrointestinal or haematological in nature
Adverse events were managed according to the protocol. TEAE, Treatment emergent adverse events.
1. This category includes the preferred terms neutrophil count decreased and neutropenia 2. This category includes the preferred terms hamoglobin decreased, red blood cell count decreased, anemia, and hematocrit decreased 3. This category includes
the preferred terms white blood cell count decreased and leukopenia 4.Tthis category includes platelet count decreased and thrombocytopenia 5. This category includes the preferred terms fatigue, asthenia, and malaise 6. Grade 1 alopecia: T-DXd =
26.5%, T-DM1 = 2.3%; grade 2, T-DXd = 9.3%.View entire presentation