AstraZeneca Investor Day Presentation Deck
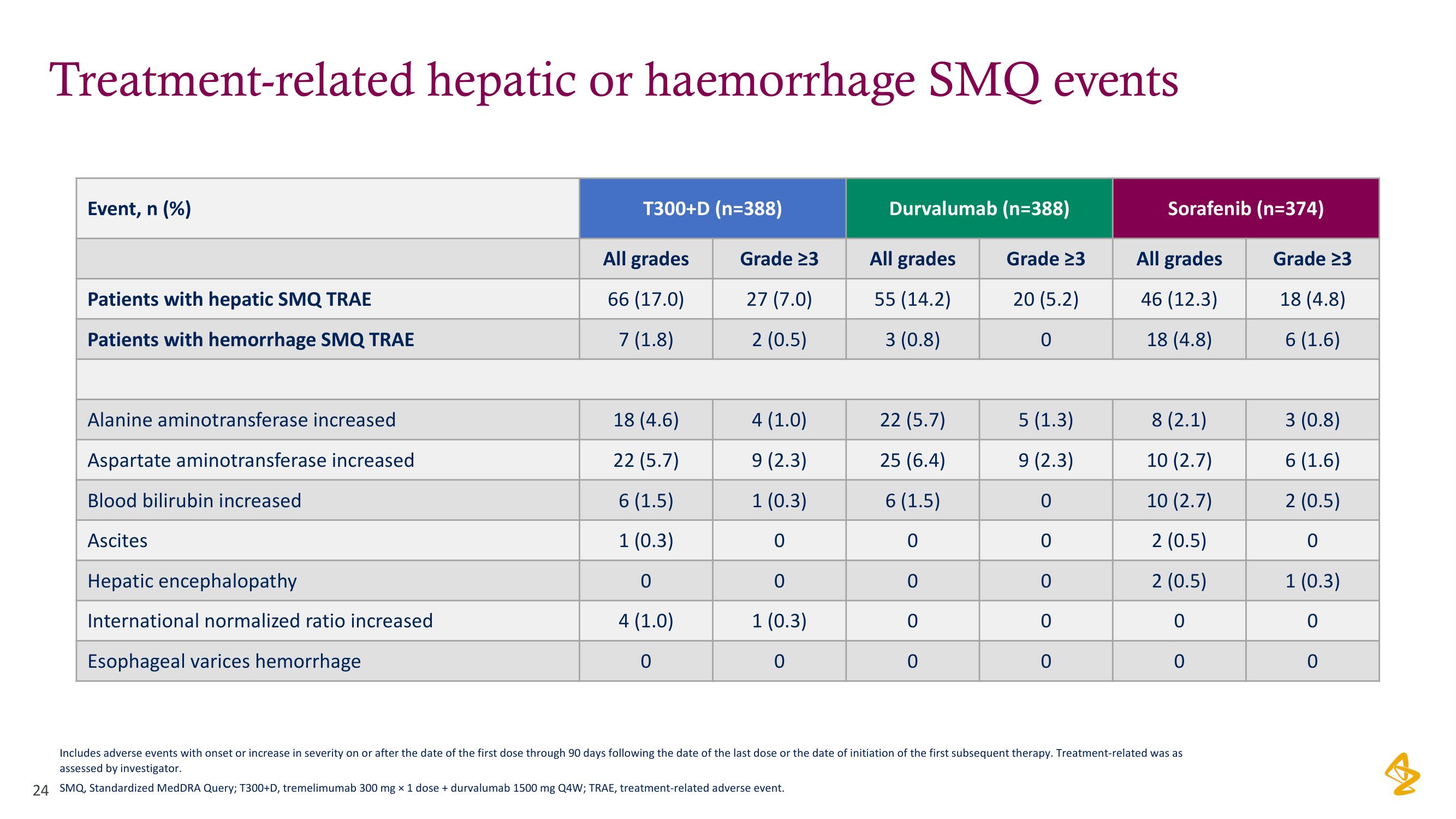
Treatment-related hepatic or haemorrhage SMQ events
Event, n (%)
Patients with hepatic SMQ TRAE
Patients with hemorrhage SMQ TRAE
Alanine aminotransferase increased
Aspartate aminotransferase increased
Blood bilirubin increased
Ascites
Hepatic encephalopathy
International normalized ratio increased
Esophageal varices hemorrhage
T300+D (n=388)
All grades
66 (17.0)
7 (1.8)
18 (4.6)
22 (5.7)
6 (1.5)
1 (0.3)
0
4 (1.0)
0
Grade 23
27 (7.0)
2 (0.5)
4 (1.0)
9 (2.3)
1 (0.3)
0
0
1 (0.3)
0
Durvalumab (n=388)
Grade 23
20 (5.2)
0
All grades
55 (14.2)
3 (0.8)
22 (5.7)
25 (6.4)
6 (1.5)
0
0
0
0
5 (1.3)
9 (2.3)
0
0
0
0
0
Sorafenib (n=374)
All grades
46 (12.3)
18 (4.8)
8 (2.1)
10 (2.7)
10 (2.7)
2 (0.5)
2 (0.5)
0
0
Includes adverse events with onset or increase in severity on or after the date of the first dose through 90 days following the date of the last dose or the date of initiation of the first subsequent therapy. Treatment-related was as
assessed by investigator.
24 SMQ, Standardized MedDRA Query; T300+D, tremelimumab 300 mg x 1 dose + durvalumab 1500 mg Q4W; TRAE, treatment-related adverse event.
Grade 23
18 (4.8)
6 (1.6)
3 (0.8)
6 (1.6)
2 (0.5)
0
1 (0.3)
0
0
3View entire presentation