AstraZeneca Investor Day Presentation Deck
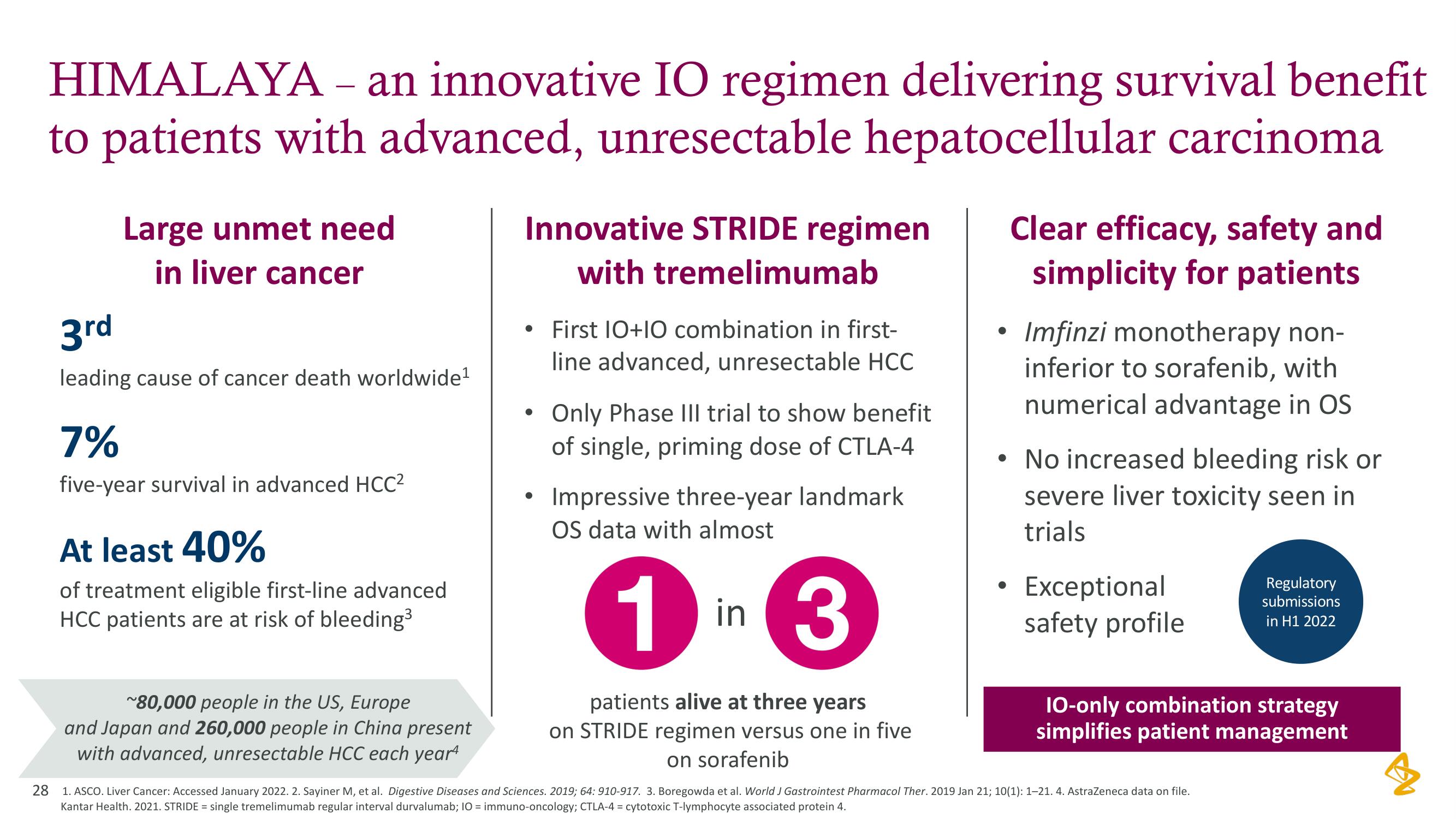
HIMALAYA - an innovative IO regimen delivering survival benefit
to patients with advanced, unresectable hepatocellular carcinoma
Large unmet need
in liver cancer
3rd
leading cause of cancer death worldwide¹
7%
five-year survival in advanced HCC²
At least 40%
of treatment eligible first-line advanced
HCC patients are at risk of bleeding³
~80,000 people in the US, Europe
and Japan and 260,000 people in China present
with advanced, unresectable HCC each year
Innovative STRIDE regimen
with tremelimumab
• First 10+10 combination in first-
line advanced, unresectable HCC
Only Phase III trial to show benefit
of single, priming dose of CTLA-4
Impressive three-year landmark
OS data with almost
1 in
in 3
patients alive at three years
on STRIDE regimen versus one in five
on sorafenib
●
Clear efficacy, safety and
simplicity for patients
Imfinzi monotherapy non-
inferior to sorafenib, with
numerical advantage in OS
• No increased bleeding risk or
severe liver toxicity seen in
trials
Exceptional
safety profile
Regulatory
submissions
in H1 2022
10-only combination strategy
simplifies patient management
28
1. ASCO. Liver Cancer: Accessed January 2022. 2. Sayiner M, et al. Digestive Diseases and Sciences. 2019; 64: 910-917. 3. Boregowda et al. World J Gastrointest Pharmacol Ther. 2019 Jan 21; 10(1): 1-21. 4. AstraZeneca data on file.
Kantar Health. 2021. STRIDE = single tremelimumab regular interval durvalumab; 10 = immuno-oncology; CTLA-4 = cytotoxic T-lymphocyte associated protein 4.View entire presentation