AstraZeneca Results Presentation Deck
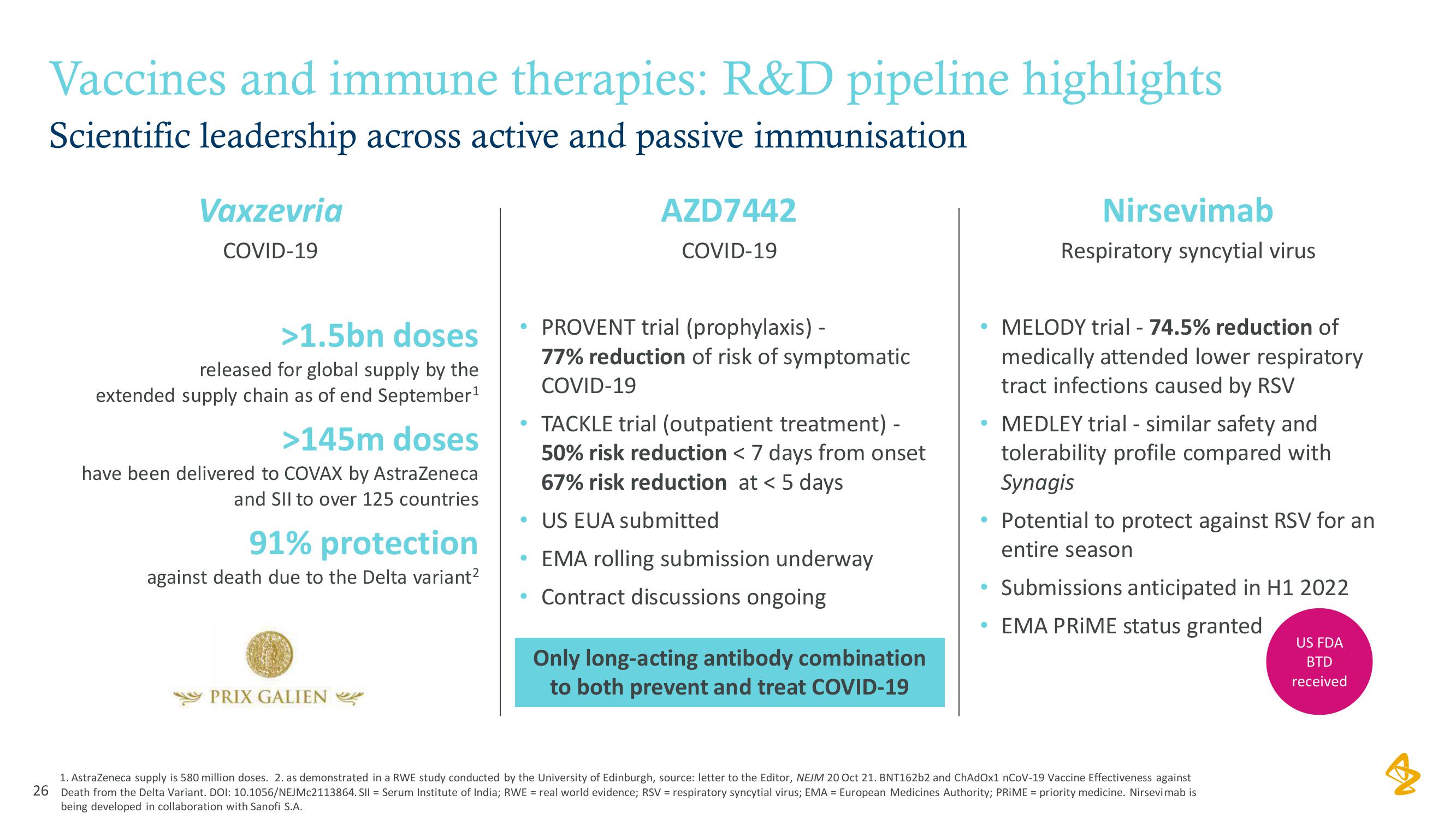
Vaccines and immune therapies: R&D pipeline highlights
Scientific leadership across active and passive immunisation
Vaxzevria
COVID-19
>1.5bn doses
released for global supply by the
extended supply chain as of end September ¹
>145m doses
have been delivered to COVAX by AstraZeneca
and SII to over 125 countries
91% protection
against death due to the Delta variant²
PRIX GALIEN
AZD7442
COVID-19
PROVENT trial (prophylaxis) -
77% reduction of risk of symptomatic
COVID-19
TACKLE trial (outpatient treatment) -
50% risk reduction < 7 days from onset
67% risk reduction at < 5 days
• US EUA submitted
EMA rolling submission underway
• Contract discussions ongoing
Only long-acting antibody combination
to both prevent and treat COVID-19
Nirsevimab
Respiratory syncytial virus
MELODY trial - 74.5% reduction of
medically attended lower respiratory
tract infections caused by RSV
• MEDLEY trial - similar safety and
tolerability profile compared with
Synagis
• Potential to protect against RSV for an
entire season
Submissions anticipated in H1 2022
EMA PRIME status granted
1. AstraZeneca supply is 580 million doses. 2. as demonstrated in a RWE study conducted by the University of Edinburgh, source: letter to the Editor, NEJM 20 Oct 21. BNT162b2 and ChAdOx1 nCoV-19 Vaccine Effectiveness against
26 Death from the Delta Variant. DOI: 10.1056/NEJMC2113864. SII = Serum Institute of India; RWE = real world evidence; RSV = respiratory syncytial virus; EMA = European Medicines Authority; PRIME = priority medicine. Nirsevimab is
being developed in collaboration with Sanofi S.A.
US FDA
BTD
received
3View entire presentation