Chimerix Investor Conference Presentation Deck
N
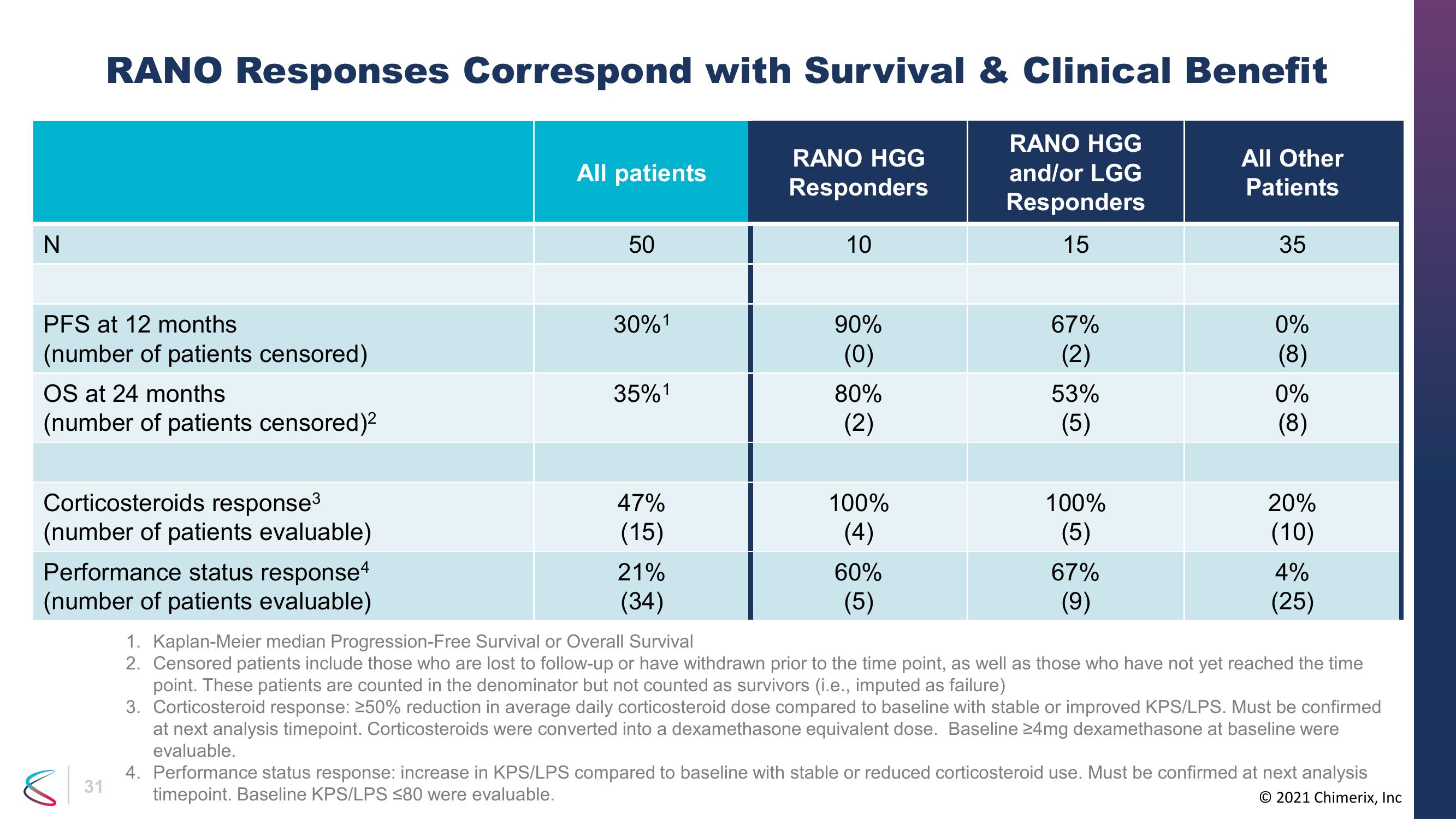
RANO Responses Correspond with Survival & Clinical Benefit
RANO HGG
and/or LGG
Responders
15
PFS at 12 months
(number of patients censored)
OS at 24 months
(number of patients censored)²
Corticosteroids response³
(number of patients evaluable)
Performance status response4
(number of patients evaluable)
✓
All patients
50
30%¹
35%1
47%
(15)
21%
(34)
RANO HGG
Responders
10
90%
(0)
80%
(2)
100%
(4)
60%
(5)
67%
(2)
53%
(5)
100%
(5)
67%
(9)
All Other
Patients
35
0%
(8)
0%
(8)
20%
(10)
4%
(25)
1. Kaplan-Meier median Progression-Free Survival or Overall Survival
2. Censored patients include those who are lost to follow-up or have withdrawn prior to the time point, as well as those who have not yet reached the time
point. These patients are counted in the denominator but not counted as survivors (i.e., imputed as failure)
3. Corticosteroid response: ≥50% reduction in average daily corticosteroid dose compared to baseline with stable or improved KPS/LPS. Must be confirmed
at next analysis timepoint. Corticosteroids were converted into a dexamethasone equivalent dose. Baseline 24mg dexamethasone at baseline were
evaluable.
4. Performance status response: increase in KPS/LPS compared to baseline with stable or reduced corticosteroid use. Must be confirmed at next analysis
31
timepoint. Baseline KPS/LPS ≤80 were evaluable.
© 2021 Chimerix, IncView entire presentation