AstraZeneca Investor Day Presentation Deck
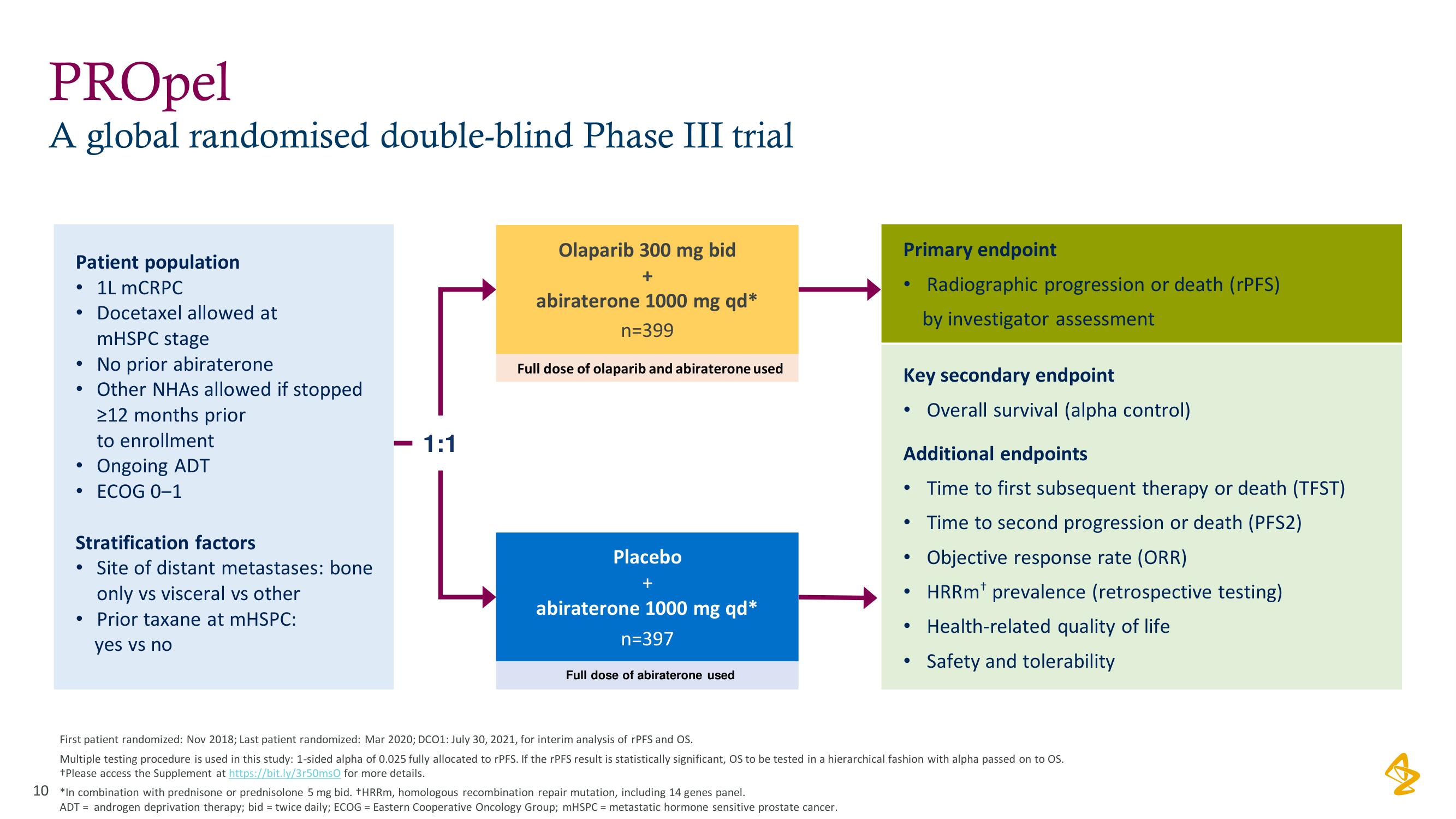
PROpel
A global randomised double-blind Phase III trial
Patient population
• 1L mCRPC
●
●
●
●
Docetaxel allowed at
mHSPC stage
●
No prior abiraterone
Other NHAs allowed if stopped
>12 months prior
to enrollment
Ongoing ADT
ECOG 0-1
Stratification factors
Site of distant metastases: bone
only vs visceral vs other
• Prior taxane at mHSPC:
yes vs no
- 1:1
Olaparib 300 mg bid
+
abiraterone 1000 mg qd*
n=399
Full dose of olaparib and abiraterone used
Placebo
+
abiraterone 1000 mg qd*
n=397
Full dose of abiraterone used
Primary endpoint
Radiographic progression or death (rPFS)
by investigator assessment
10 *In combination with prednisone or prednisolone 5 mg bid. +HRRm, homologous recombination repair mutation, including 14 genes panel.
ADT androgen deprivation therapy; bid = twice daily; ECOG = Eastern Cooperative Oncology Group; mHSPC = metastatic hormone sensitive prostate cancer.
●
Key secondary endpoint
Overall survival (alpha control)
Additional endpoints
Time to first subsequent therapy or death (TFST)
Time to second progression or death (PFS2)
Objective response rate (ORR)
HRRm¹ prevalence (retrospective testing)
Health-related quality of life
Safety and tolerability
●
●
●
First patient randomized: Nov 2018; Last patient randomized: Mar 2020; DCO1: July 30, 2021, for interim analysis of rPFS and OS.
Multiple testing procedure is used in this study: 1-sided alpha of 0.025 fully allocated to rPFS. If the rPFS result is statistically significant, OS to be tested in a hierarchical fashion with alpha passed on to OS.
+Please access the Supplement at https://bit.ly/3r50ms0 for more details.
BView entire presentation