BioNTech Investor Day Presentation Deck
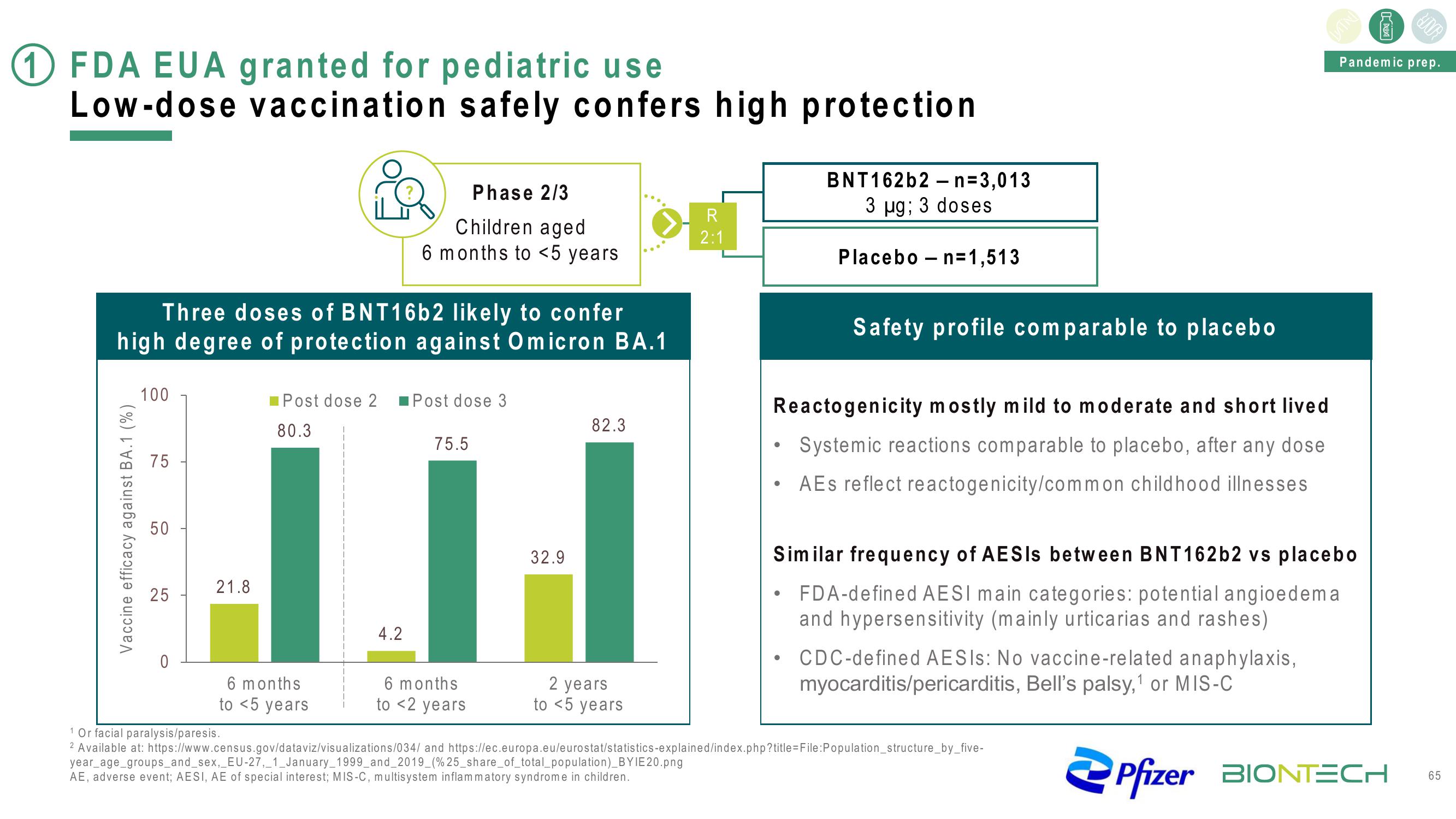
1 FDA EUA granted for pediatric use
Low-dose vaccination safely confers high protection
Three doses of BNT16b2 likely to confer
high degree of protection against Omicron BA.1
Vaccine efficacy against BA.1 (%)
100
75
50
25
0
21.8
Phase 2/3
Children aged
6 months to <5 years
Post dose 2 Post dose 3
80.3
6 months
to <5 years
4.2
75.5
6 months
to <2 years
32.9
82.3
2 years
to <5 years
R
2:1
year_age_groups_and_sex,_EU-27,_1_January_1999_and_2019_(%25_share_of_total_population)_BYIE 20.png
AE, adverse event; AESI, AE of special interest; MIS-C, multisystem inflammatory syndrome in children.
BNT162b2n=3,013
3 µg; 3 doses
Placebo n=1,513
Safety profile comparable to placebo
Reactogenicity mostly mild to moderate and short lived
Systemic reactions comparable to placebo, after any dose
AEs reflect reactogenicity/common childhood illnesses
1 Or facial paralysis/paresis.
2 Available at: https://www.census.gov/dataviz/visualizations/034/ and https://ec.europa.eu/eurostat/statistics -explained/index.php?title=File:Population structure_by_five-
Similar frequency of AESIs between BNT162b2 vs placebo
• FDA-defined AESI main categories: potential angioedema
and hypersensitivity (mainly urticarias and rashes)
CDC-defined AESIS: No vaccine-related anaphylaxis,
myocarditis/pericarditis, Bell's palsy,¹ or MIS-C
[DAN
Pandemic prep.
CAR
Pfizer BIONTECH
Pfizer
65View entire presentation