Company Overview
For personal use only
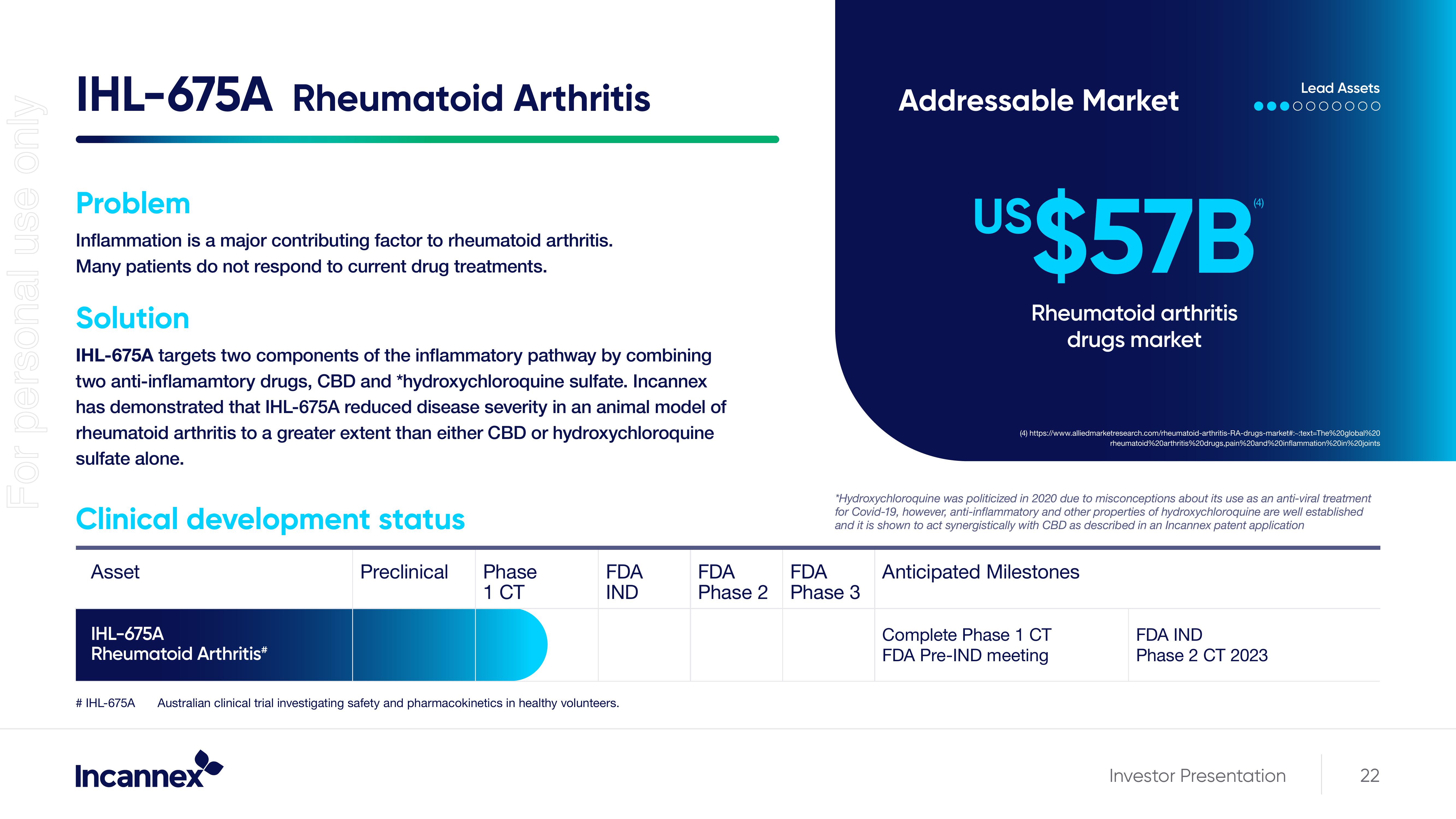
IHL-675A Rheumatoid Arthritis
Problem
Inflammation is a major contributing factor to rheumatoid arthritis.
Many patients do not respond to current drug treatments.
Solution
IHL-675A targets two components of the inflammatory pathway by combining
two anti-inflamamtory drugs, CBD and *hydroxychloroquine sulfate. Incannex
has demonstrated that IHL-675A reduced disease severity in an animal model of
rheumatoid arthritis to a greater extent than either CBD or hydroxychloroquine
sulfate alone.
Clinical development status
Asset
IHL-675A
Rheumatoid Arthritis#
Preclinical Phase
1 CT
Incannex
FDA
IND
# IHL-675A Australian clinical trial investigating safety and pharmacokinetics in healthy volunteers.
FDA
Phase 2
Addressable Market
FDA
Phase 3
Lead Assets
0000000000
US$57B
Rheumatoid arthritis
drugs market
(4) https://www.alliedmarketresearch.com/rheumatoid-arthritis-RA-drugs-market#:~.text-The%20global%20
*Hydroxychloroquine was politicized in 2020 due to misconceptions about its use as an anti-viral treatment
for Covid-19, however, anti-inflammatory and other properties of hydroxychloroquine are well established
and it is shown to act synergistically with CBD as described in an Incannex patent application
Anticipated Milestones
Complete Phase 1 CT
FDA Pre-IND meeting
rheumatoid%20arthritis%20drugs,pain%20and%20inflammation%20in%20joints
FDA IND
Phase 2 CT 2023
Investor Presentation
22View entire presentation