BioAtla Investor Presentation Deck
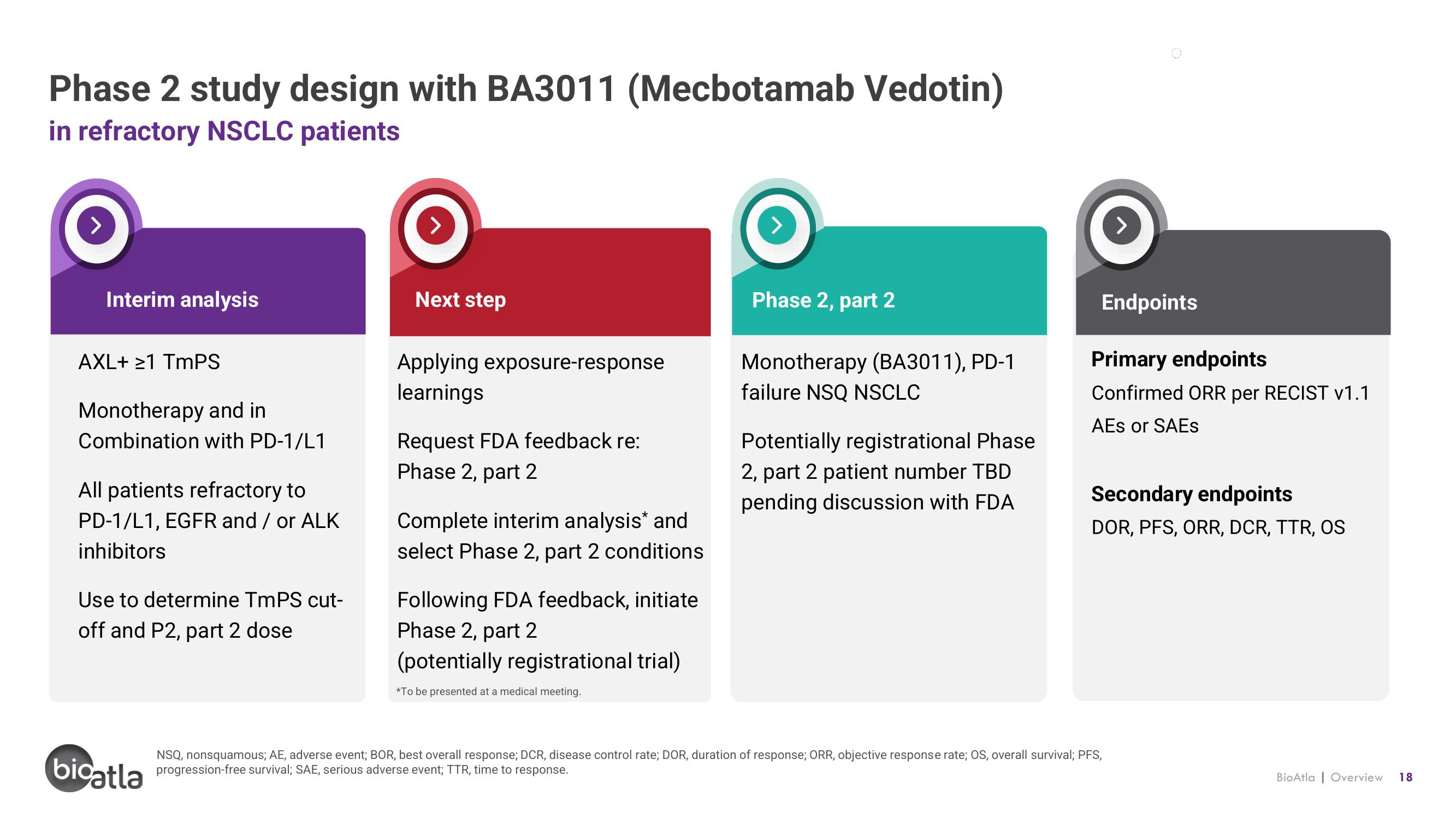
Phase 2 study design with BA3011 (Mecbotamab Vedotin)
in refractory NSCLC patients
Interim analysis
AXL+ ≥1 TmPS
Monotherapy and in
Combination with PD-1/L1
All patients refractory to
PD-1/L1, EGFR and / or ALK
inhibitors
Use to determine TmPS cut-
off and P2, part 2 dose
Next step
Applying exposure-response
learnings
Request FDA feedback re:
Phase 2, part 2
Complete interim analysis* and
select Phase 2, part 2 conditions
Following FDA feedback, initiate
Phase 2, part 2
(potentially registrational trial)
*To be presented at a medical meeting.
Phase 2, part 2
bicatla progression-free survival; SAE, serious adverse event; TTR, time to response.
Monotherapy (BA3011), PD-1
failure NSQ NSCLC
Potentially registrational Phase
2, part 2 patient number TBD
pending discussion with FDA
Endpoints
Primary endpoints
Confirmed ORR per RECIST v1.1
AES or SAEs
Secondary endpoints
DOR, PFS, ORR, DCR, TTR, OS
NSQ, nonsquamous; AE, adverse event; BOR, best overall response; DCR, disease control rate; DOR, duration of response; ORR, objective response rate; OS, overall survival; PFS,
BioAtla| Overview 18View entire presentation