BioNTech Investor Day Presentation Deck
Proportion of patients (%)
Fix Vac I BNT111
Strong immunogenicity and promising clinical activity in Phase 1 Lipo-MERIT
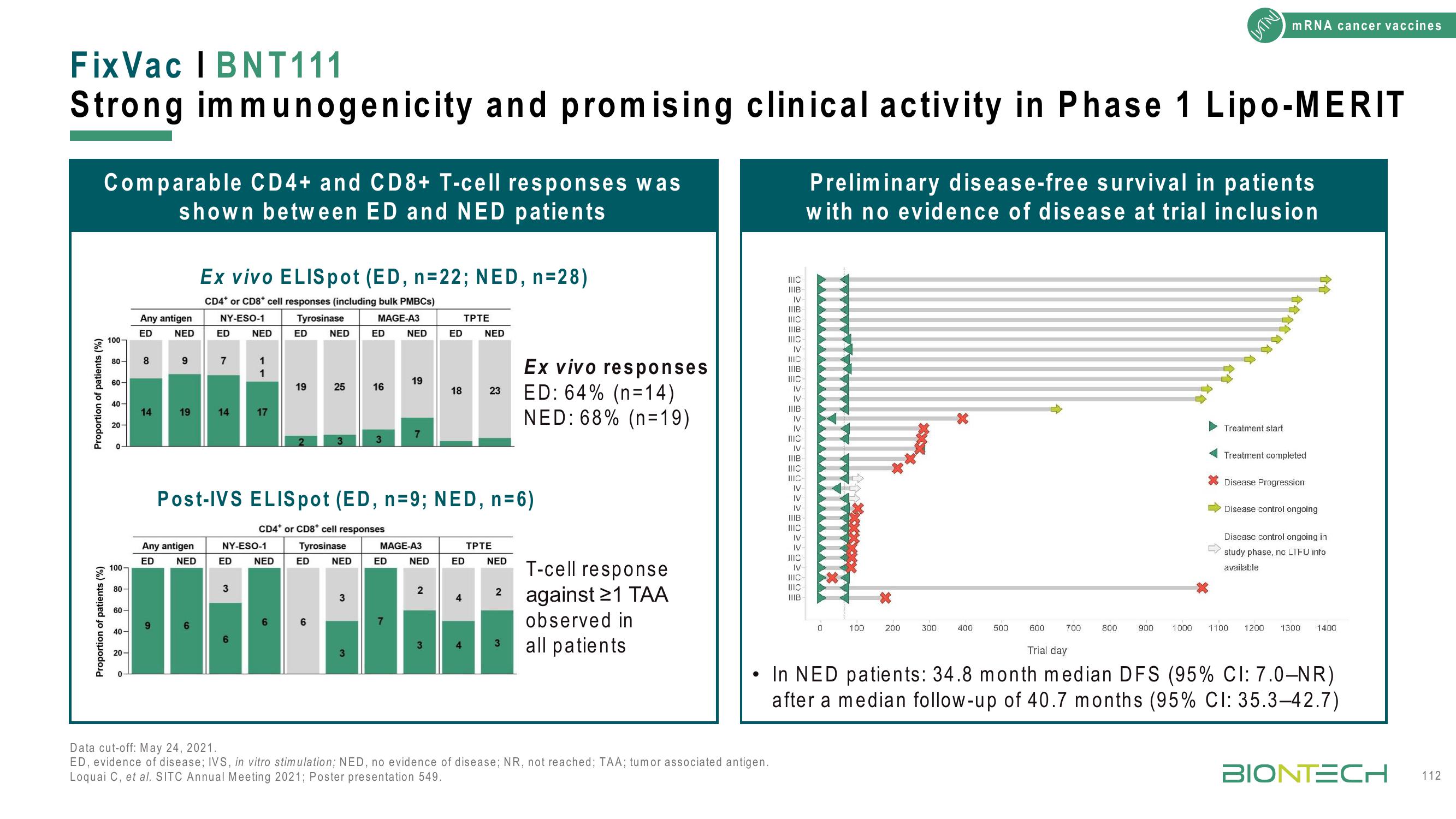
Comparable CD4+ and CD8+ T-cell responses was
shown between ED and NED patients
Proportion of patients (%)
100
80- 8
60
40
20
100
80-
60-
40-
20-
Any antigen
ED
NED
0
14
9
9
19
Any antigen
ED
NED
Ex vivo ELISpot (ED, n=22; NED, n=28)
CD4* or CD8+ cell responses (including bulk PMBCs)
NY-ESO-1
Tyrosinase
MAGE-A3
6
ED
7
14
NED
ED
1
1
3
17
NY-ESO-1
NED
ED
6
19
ED
NED
6
25
3
Post-IVS ELISpot (ED, n=9; NED, n=6)
CD4* or CD8+ cell responses
Tyrosinase
NED
3
ED
3
16
NED
19
ED
MAGE-A3
NED
ED
2
18
TPTE
ED
4
4
NED
23
TPTE
NED
2
Ex vivo responses
ED: 64% (n=14)
NED: 68% (n=19)
3
T-cell response
against 21 TAA
observed in
all patients
●
Data cut-off: May 24, 2021.
ED, evidence of disease; IVS, in vitro stimulation; NED, no evidence of disease; NR, not reached; TAA; tumor associated antigen.
Loquai C, et al. SITC Annual Meeting 2021; Poster presentation 549.
IIIC
IIIB
IV
IIIB
IIIC
IIIB
IIIC
IV
IIIC
IIIB
IIIC
IV
IV
IIIB
IV
IV
IIIC
IV
IIIB
I|IC
IIIC
IV
IV
IV
IIIB
言
IIIC
IV
IV
IIIC
IV
IIIC
IIIC
IIIB
****
Preliminary disease-free survival in patients
with no evidence of disease at trial inclusion
0
100
200
300
400
500
600
700
NUM
800
900
mRNA cancer vaccines
Treatment start
Treatment completed
Disease Progression
Disease control ongoing
Disease control ongoing in
study phase, no LTFU info
available
1000 1100 1200 1300 1400
Trial day
In NED patients: 34.8 month median DFS (95% CI: 7.0-NR)
after a median follow-up of 40.7 months (95% CI: 35.3-42.7)
BIONTECH
112View entire presentation