Ocuphire Pharma Investor Updates
P
32
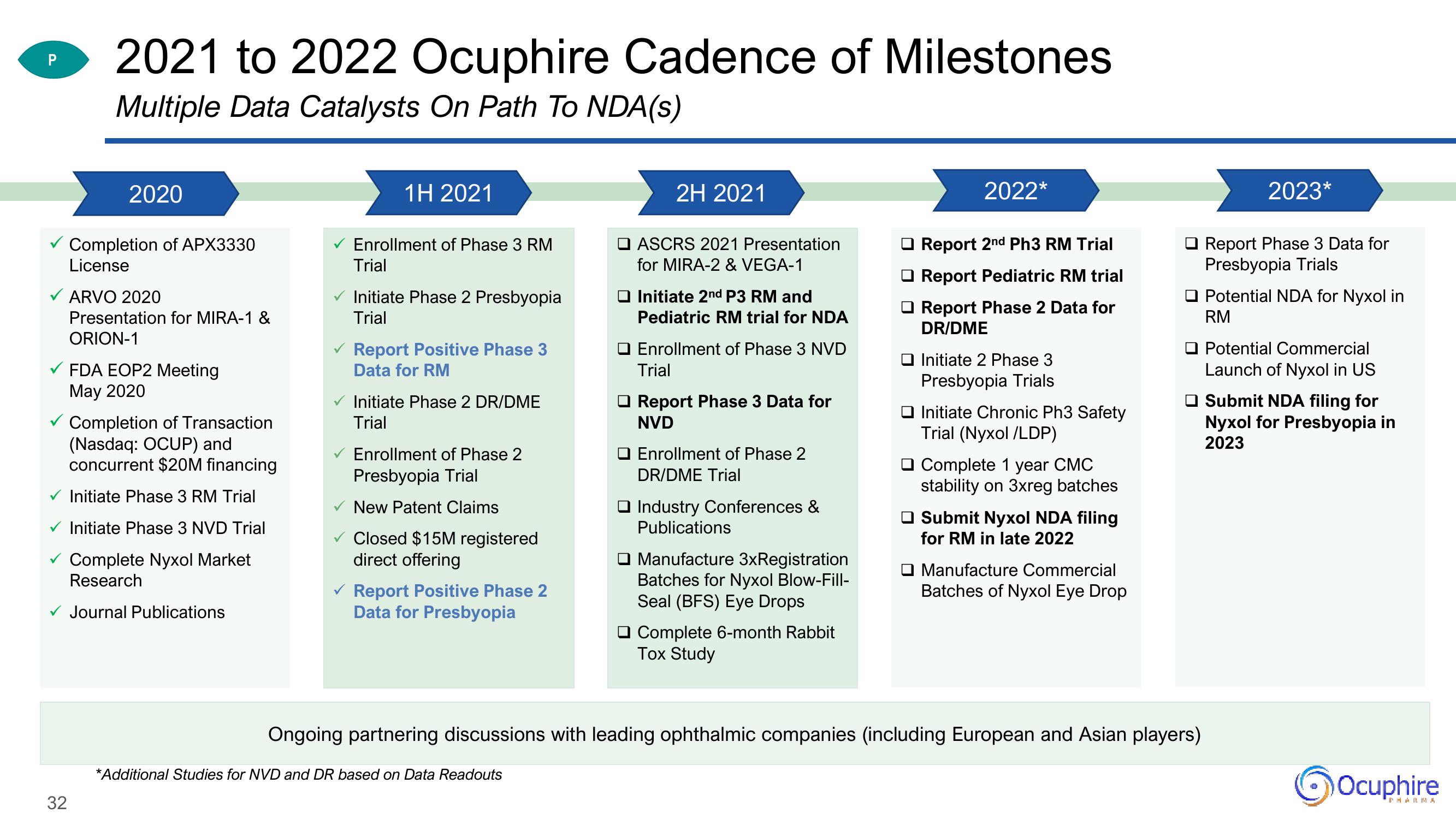
2021 to 2022 Ocuphire Cadence of Milestones
Multiple Data Catalysts On Path To NDA(s)
2020
Completion of APX3330
License
ARVO 2020
Presentation for MIRA-1 &
ORION-1
FDA EOP2 Meeting
May 2020
Completion of Transaction
(Nasdaq: OCUP) and
concurrent $20M financing
Initiate Phase 3 RM Trial
Initiate Phase 3 NVD Trial
Complete Nyxol Market
Research
Journal Publications
1H 2021
Enrollment of Phase 3 RM
Trial
Initiate Phase 2 Presbyopia
Trial
Report Positive Phase 3
Data for RM
Initiate Phase 2 DR/DME
Trial
Enrollment of Phase 2
Presbyopia Trial
New Patent Claims
Closed $15M registered
direct offering
Report Positive Phase 2
Data for Presbyopia
2H 2021
*Additional Studies for NVD and DR based on Data Readouts
ASCRS 2021 Presentation
for MIRA-2 & VEGA-1
Initiate 2nd P3 RM and
Pediatric RM trial for NDA
O Enrollment of Phase 3 NVD
Trial
Report Phase 3 Data for
NVD
Enrollment of Phase 2
DR/DME Trial
Industry Conferences &
Publications
Manufacture 3xRegistration
Batches for Nyxol Blow-Fill-
Seal (BFS) Eye Drops
Complete 6-month Rabbit
Tox Study
2022*
Report 2nd Ph3 RM Trial
Report Pediatric RM trial
Report Phase 2 Data for
DR/DME
Initiate 2 Phase 3
Presbyopia Trials
Initiate Chronic Ph3 Safety
Trial (Nyxol/LDP)
Complete 1 year CMC
stability on 3xreg batches
Submit Nyxol NDA filing
for RM in late 2022
Manufacture Commercial
Batches of Nyxol Eye Drop
Ongoing partnering discussions with leading ophthalmic companies (including European and Asian players)
2023*
Report Phase 3 Data for
Presbyopia Trials
Potential NDA for Nyxol in
RM
Potential Commercial
Launch of Nyxol in US
Submit NDA filing for
Nyxol for Presbyopia in
2023
Ocuphire
PHARMAView entire presentation