Imara M&A
●
●
ELVN-001: Despite Great Advances, a Significant Need Remains for Better
Treatment Options for Chronic Myeloid Leukemia
Challenges with Current Standard of Care
Approximately 1 in 5 patients switch therapy within the first year
and ~40% of patients switch in the first 5 years (1L & 2L)
Growing 3L+ patient population (>25% of CP-CML) with limited
treatment options
Except for asciminib, approved TKIs have poor kinase selectivity,
resulting in tolerability issues that impact efficacy
• Comorbidities, restrictions with concomitant medications,
and specific administration requirements impede long-term
patient adherence
• Fewer than 10% of patients successfully achieve sustained
treatment-free remission (TFR)
Majority of HCPs (77%) indicated need for
more effective, safe, and tolerable agents in CML
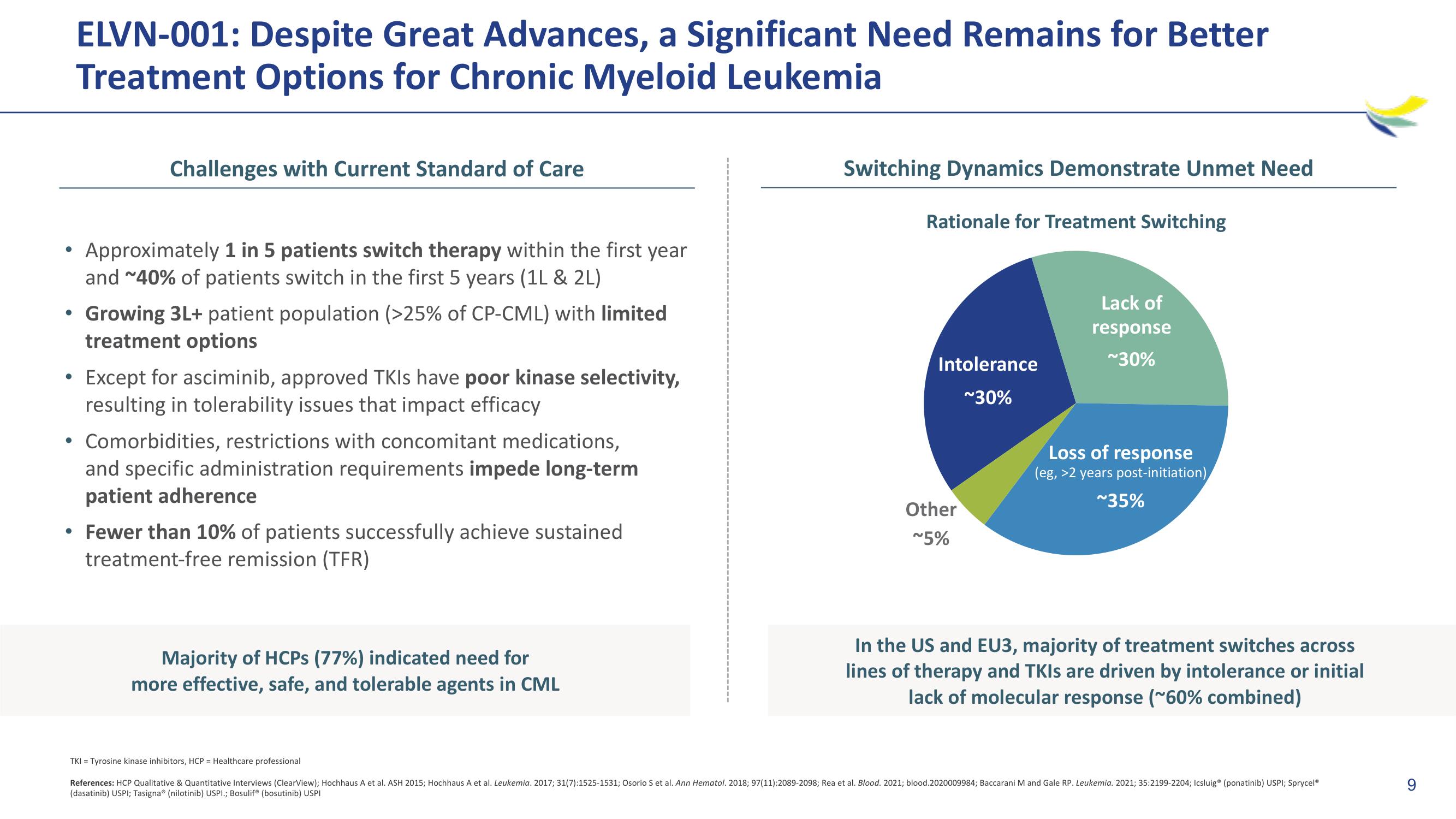
Switching Dynamics Demonstrate Unmet Need
Rationale for Treatment Switching
Intolerance
~30%
Other
~5%
Lack of
response
~30%
Loss of response
(eg, >2 years post-initiation)
~35%
In the US and EU3, majority of treatment switches across
lines of therapy and TKIs are driven by intolerance or initial
lack of molecular response (~60% combined)
TKI = Tyrosine kinase inhibitors, HCP= Healthcare professional
References: HCP Qualitative & Quantitative Interviews (ClearView); Hochhaus A et al. ASH 2015; Hochhaus A et al. Leukemia. 2017; 31(7):1525-1531; Osorio S et al. Ann Hematol. 2018; 97(11):2089-2098; Rea et al. Blood. 2021; blood.2020009984; Baccarani M and Gale RP. Leukemia. 2021; 35:2199-2204; Icsluig® (ponatinib) USPI; Sprycel®
(dasatinib) USPI; Tasigna (nilotinib) USPI.; Bosulife (bosutinib) USPI
9View entire presentation