Aravive Investor Presentation Deck
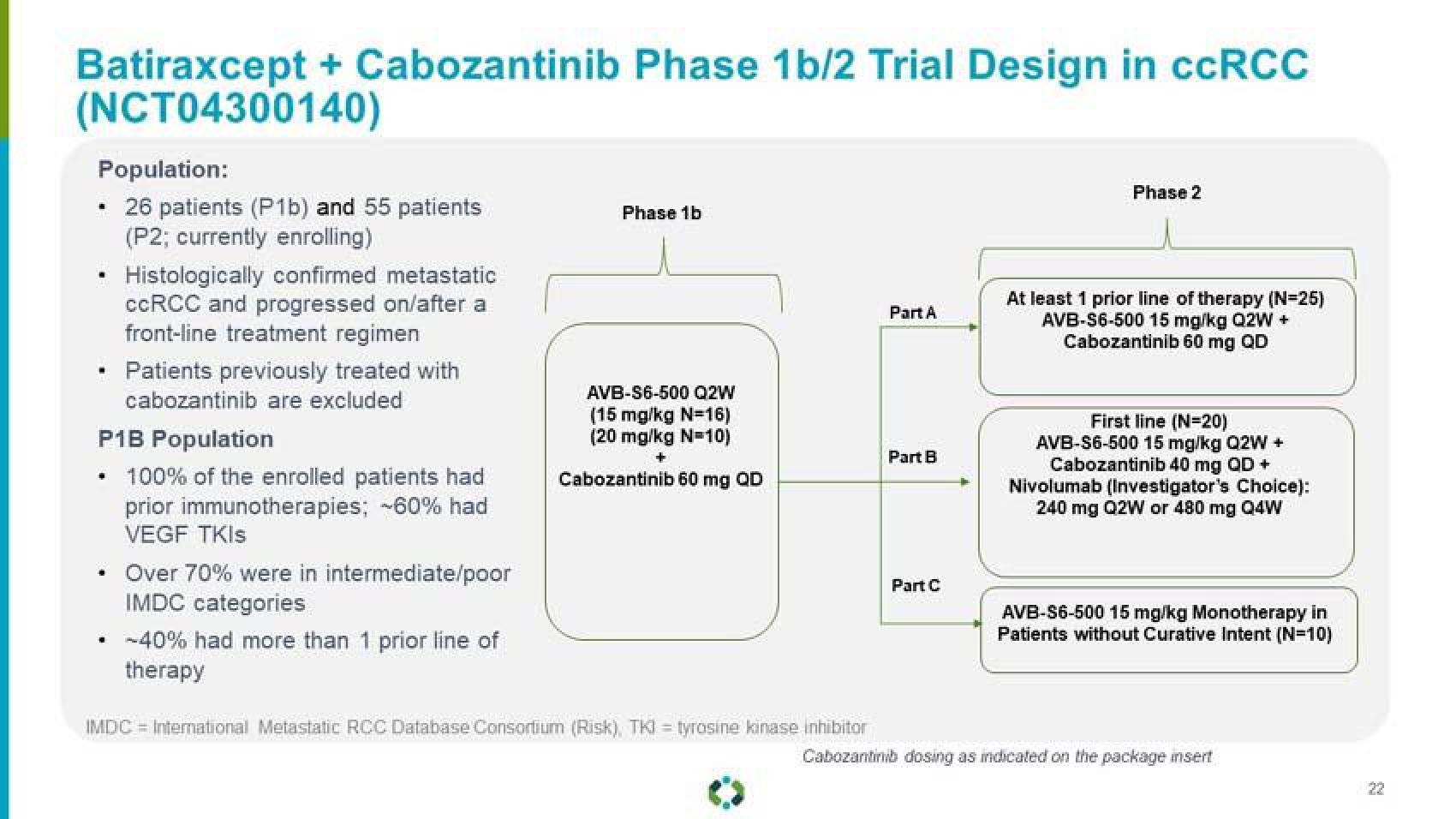
Batiraxcept + Cabozantinib Phase 1b/2 Trial Design in ccRCC
(NCT04300140)
Population:
26 patients (P1b) and 55 patients
(P2; currently enrolling)
#
.
Histologically confirmed metastatic
ccRCC and progressed on/after a
front-line treatment regimen
Patients previously treated with
cabozantinib are excluded
P1B Population
• 100% of the enrolled patients had
prior immunotherapies; -60% had
VEGF TKIS
Over 70% were in intermediate/poor
IMDC categories
-40% had more than 1 prior line of
therapy
Phase 1b
AVB-S6-500 Q2W
(15 mg/kg N=16)
(20 mg/kg N=10)
+
Cabozantinib 60 mg OD
IMDC = International Metastatic RCC Database Consortium (Risk), TKI = tyrosine kinase inhibitor
Part A
Part B
Part C
Phase 2
At least 1 prior line of therapy (N=25)
AVB-S6-500 15 mg/kg Q2W +
Cabozantinib 60 mg QD
First line (N=20)
AVB-S6-500 15 mg/kg Q2W +
Cabozantinib 40 mg QD +
Nivolumab (Investigator's Choice):
240 mg Q2W or 480 mg Q4W
AVB-S6-500 15 mg/kg Monotherapy in
Patients without Curative Intent (N=10)
Cabozantinib dosing as indicated on the package insert
NView entire presentation