Immix Biopharma Investor Presentation Deck
IMX-110 Preclinical Data Provides Translational Rationale & PD-1 Combination Rationale
50%
0%
(50%)
(100%)
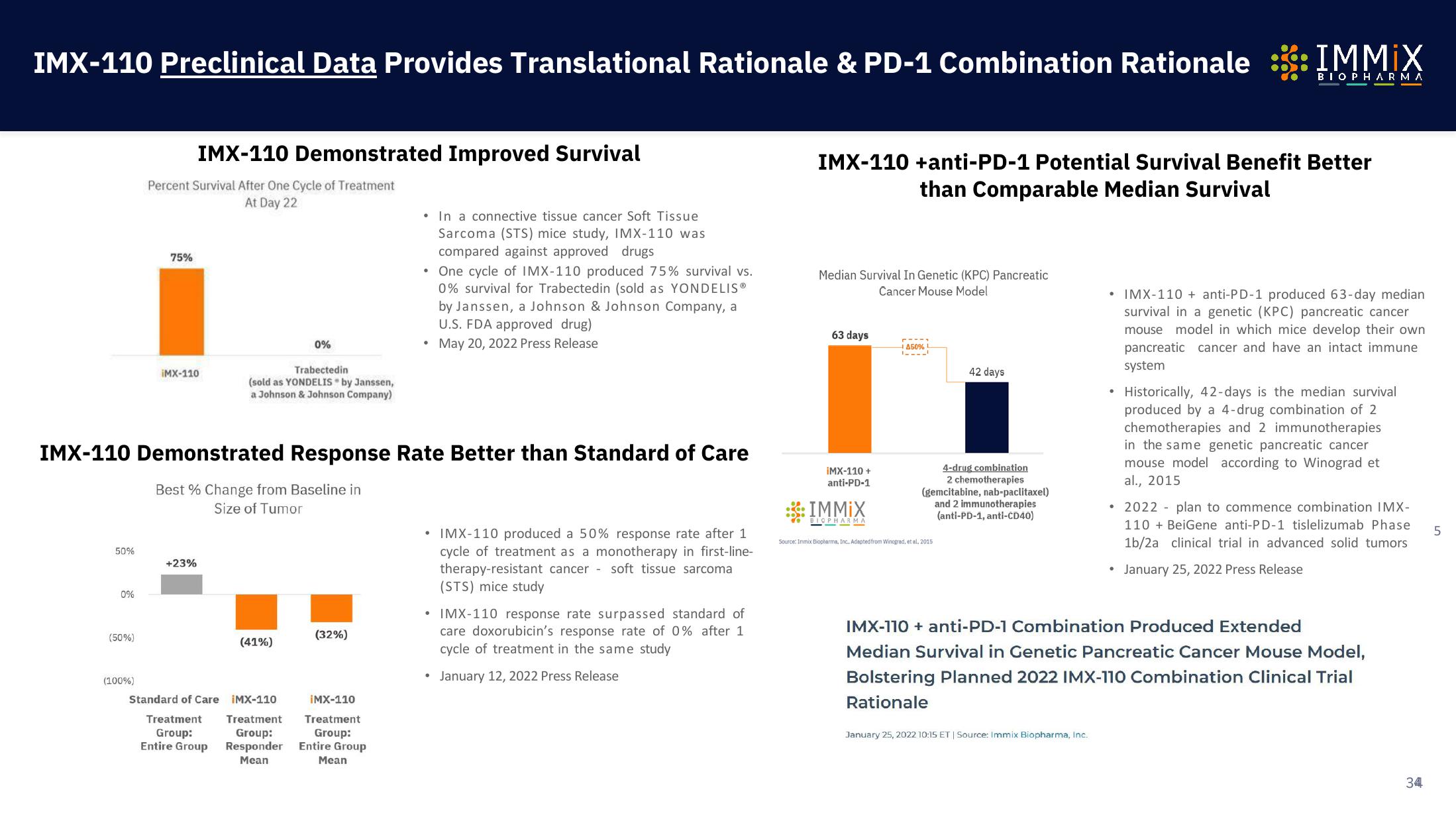
Percent Survival After One Cycle of Treatment
At Day 22
75%
IMX-110 Demonstrated Improved Survival
iMX-110
IMX-110 Demonstrated Response Rate Better than Standard of Care
Best % Change from Baseline in
Size of Tumor
+23%
Trabectedin
(sold as YONDELIS by Janssen,
a Johnson & Johnson Company)
0%
(41%)
Standard of Care IMX-110
Treatment Treatment
Group:
Group:
Responder
Entire Group
Mean
(32%)
¡MX-110
Treatment
Group:
Entire Group
Mean
In a connective tissue cancer Soft Tissue
Sarcoma (STS) mice study, IMX-110 was
compared against approved drugs
• One cycle of IMX-110 produced 75% survival vs.
0% survival for Trabectedin (sold as YONDELIS®
by Janssen, a Johnson & Johnson Company, a
U.S. FDA approved drug)
May 20, 2022 Press Release
• IMX-110 produced a 50% response rate after 1
cycle of treatment as a monotherapy in first-line-
therapy-resistant cancer soft tissue sarcoma
(STS) mice study
• IMX-110 response rate surpassed standard of
care doxorubicin's response rate of 0% after 1
cycle of treatment in the same study
January 12, 2022 Press Release
●
Median Survival In Genetic (KPC) Pancreatic
Cancer Mouse Model
IMX-110 +anti-PD-1 Potential Survival Benefit Better
than Comparable Median Survival
63 days
IMX-110 +
anti-PD-1
IMMIX
BIOPHARMA
A50%!
05070
42 days
4-drug combination
2 chemotherapies
(gemcitabine, nab-paclitaxel)
and 2 immunotherapies
(anti-PD-1, anti-CD40)
Source: Inmix Biopharma, Inc. Adapted from Winograd, et al, 2015
January 25, 2022 10:15 ET | Source: Immix Biopharma, Inc.
●●●
●
BIOPHARMA
• IMX-110 + anti-PD-1 produced 63-day median
survival in a genetic (KPC) pancreatic cancer
mouse model in which mice develop their own
pancreatic cancer and have an intact immune
system
●
Historically, 42-days is the median survival
produced by a 4-drug combination of 2
chemotherapies and 2 immunotherapies
in the same genetic pancreatic cancer
mouse model according to Winograd et
al., 2015
• 2022 plan to commence combination IMX-
110 + BeiGene anti-PD-1 tislelizumab Phase
1b/2a clinical trial in advanced solid tumors
January 25, 2022 Press Release
IMX-110 + anti-PD-1 Combination Produced Extended
Median Survival in Genetic Pancreatic Cancer Mouse Model,
Bolstering Planned 2022 IMX-110 Combination Clinical Trial
Rationale
34
5View entire presentation