Ocuphire Pharma Results Presentation Deck
28
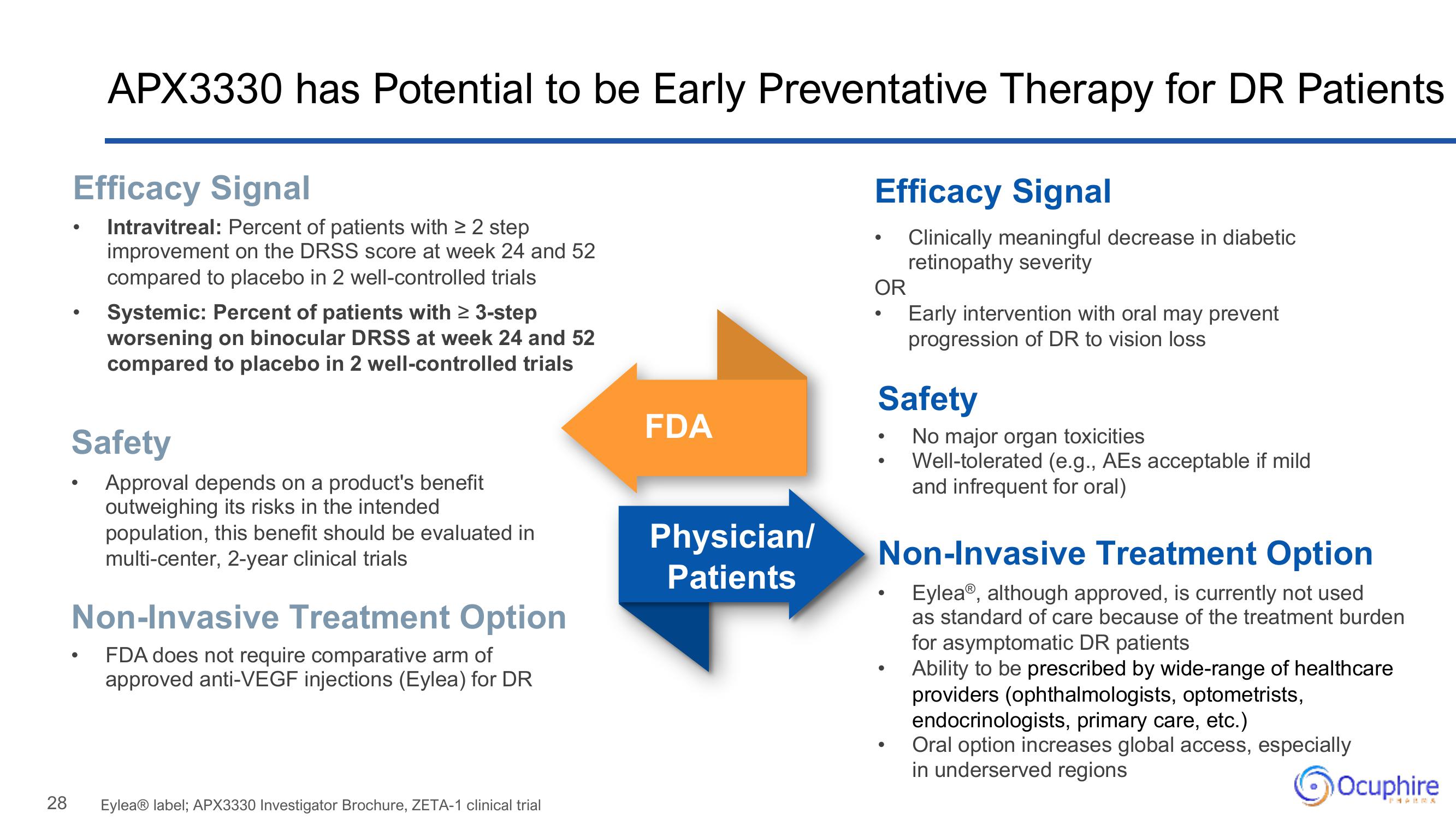
Efficacy Signal
Intravitreal: Percent of patients with ≥ 2 step
improvement on the DRSS score at week 24 and 52
compared to placebo in 2 well-controlled trials
●
●
APX3330 has Potential to be Early Preventative Therapy for DR Patients
Systemic: Percent of patients with ≥ 3-step
worsening on binocular DRSS at week 24 and 52
compared to placebo in 2 well-controlled trials
Safety
Approval depends on a product's benefit
outweighing its risks in the intended.
population, this benefit should be evaluated in
multi-center, 2-year clinical trials
●
Non-Invasive Treatment Option
FDA does not require comparative arm of
approved anti-VEGF injections (Eylea) for DR
Eylea® label; APX3330 Investigator Brochure, ZETA-1 clinical trial
FDA
Physician/
Patients
Efficacy Signal
Clinically meaningful decrease in diabetic
retinopathy severity
●
OR
Safety
●
Early intervention with oral may prevent
progression of DR to vision loss
●
No major organ toxicities
Well-tolerated (e.g., AEs acceptable if mild
and infrequent for oral)
Non-Invasive Treatment Option
Eylea®, although approved, is currently not used
as standard of care because of the treatment burden
for asymptomatic DR patients
Ability to be prescribed by wide-range of healthcare
providers (ophthalmologists, optometrists,
endocrinologists, primary care, etc.)
Oral option increases global access, especially
in underserved regions
OcuphireView entire presentation