Vaxcyte Corporate Presentation
20
20
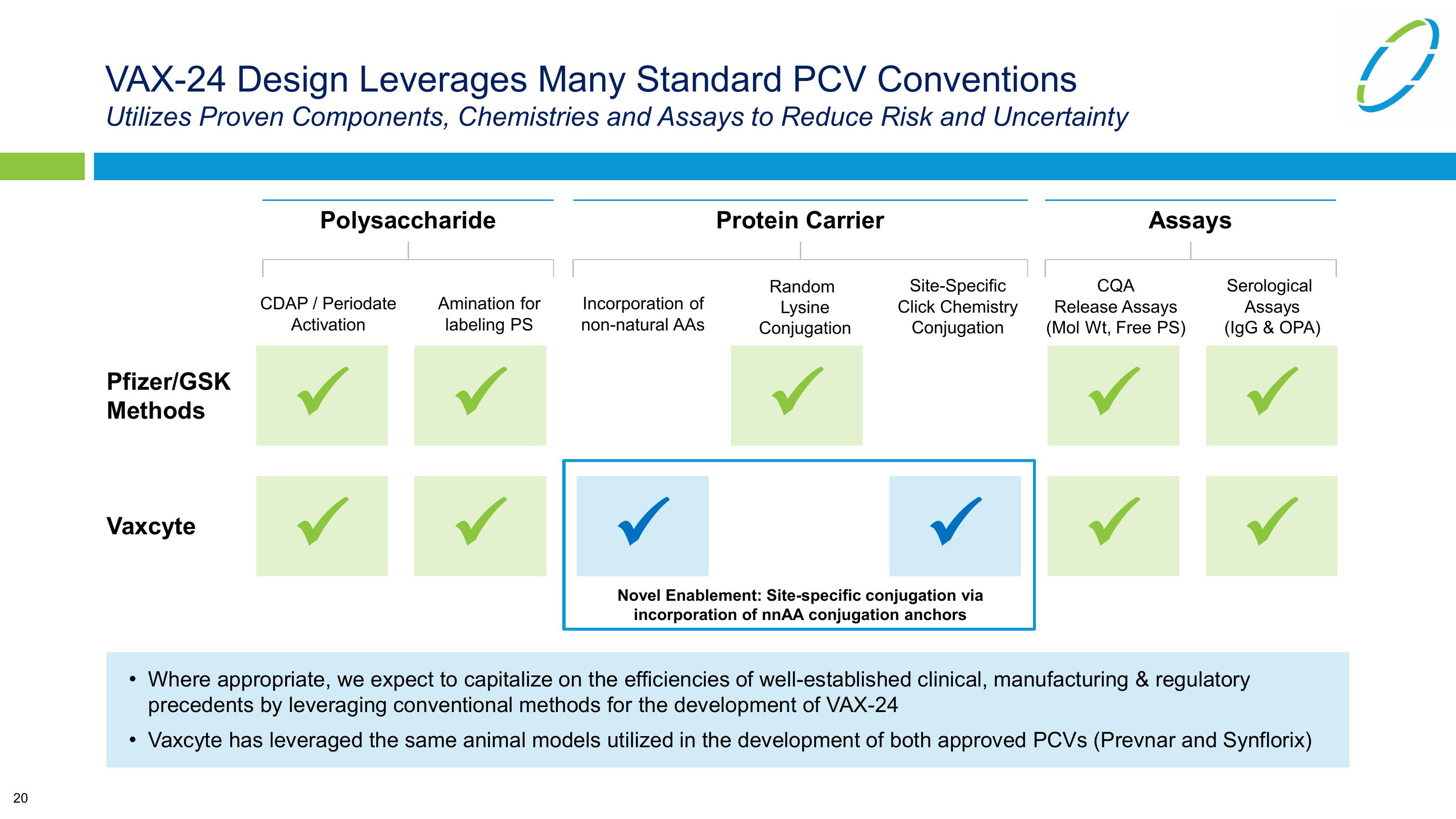
VAX-24 Design Leverages Many Standard PCV Conventions
Utilizes Proven Components, Chemistries and Assays to Reduce Risk and Uncertainty
Pfizer/GSK
Methods
Vaxcyte
Polysaccharide
Protein Carrier
CDAP / Periodate
Activation
Amination for
labeling PS
Incorporation of
non-natural AAs
Random
Lysine
Conjugation
Site-Specific
Click Chemistry
Conjugation
Novel Enablement: Site-specific conjugation via
incorporation of nnAA conjugation anchors
Assays
CQA
Release Assays
(Mol Wt, Free PS)
Serological
Assays
(IgG & OPA)
о
• Where appropriate, we expect to capitalize on the efficiencies of well-established clinical, manufacturing & regulatory
precedents by leveraging conventional methods for the development of VAX-24
.
Vaxcyte has leveraged the same animal models utilized in the development of both approved PCVs (Prevnar and Synflorix)View entire presentation