Centessa IPO Presentation Deck
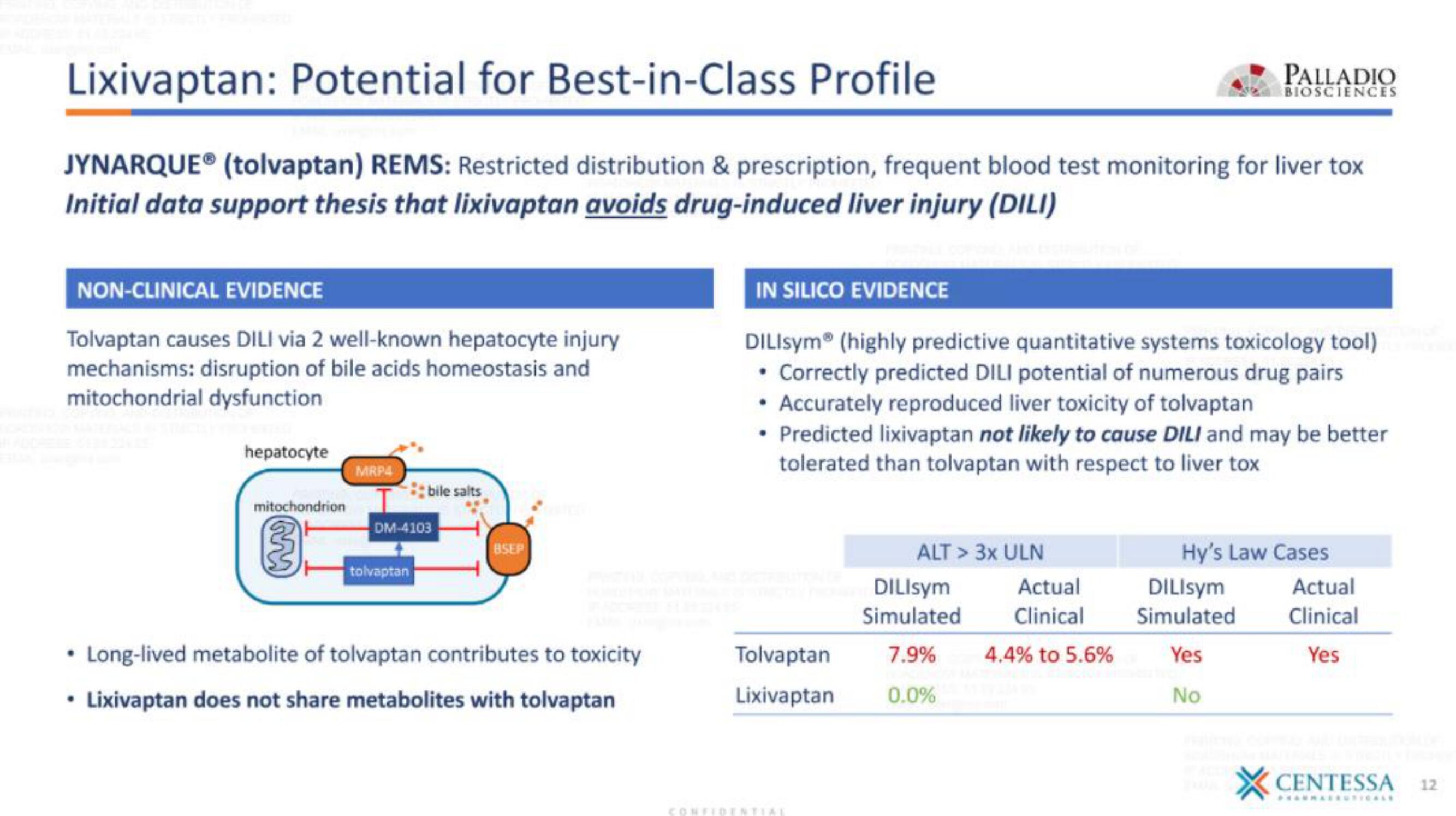
Lixivaptan: Potential for Best-in-Class Profile
JYNARQUEⓇ (tolvaptan) REMS: Restricted distribution & prescription, frequent blood test monitoring for liver tox
Initial data support thesis that lixivaptan avoids drug-induced liver injury (DILI)
NON-CLINICAL EVIDENCE
Tolvaptan causes DILI via 2 well-known hepatocyte injury
mechanisms: disruption of bile acids homeostasis and
mitochondrial dysfunction
hepatocyte
mitochondrion
MRP4
bile salts
DM-4103
tolvaptan
BSEP
• Long-lived metabolite of tolvaptan contributes to toxicity
• Lixivaptan does not share metabolites with tolvaptan
IN SILICO EVIDENCE
DILISym (highly predictive quantitative systems toxicology tool)
• Correctly predicted DILI potential of numerous drug pairs
• Accurately reproduced liver toxicity of tolvaptan
• Predicted lixivaptan not likely to cause DILI and may be better
tolerated than tolvaptan with respect to liver tox
Tolvaptan
Lixivaptan
ALT > 3x ULN
DILIsym
Simulated
7.9%
0.0%
PALLADIO
BIOSCIENCES
Actual
Clinical
4.4% to 5.6%
Hy's Law Cases
DILISym
Simulated
Yes
No
Actual
Clinical
Yes
CENTESSA 12View entire presentation