Ocuphire Pharma Results Presentation Deck
29
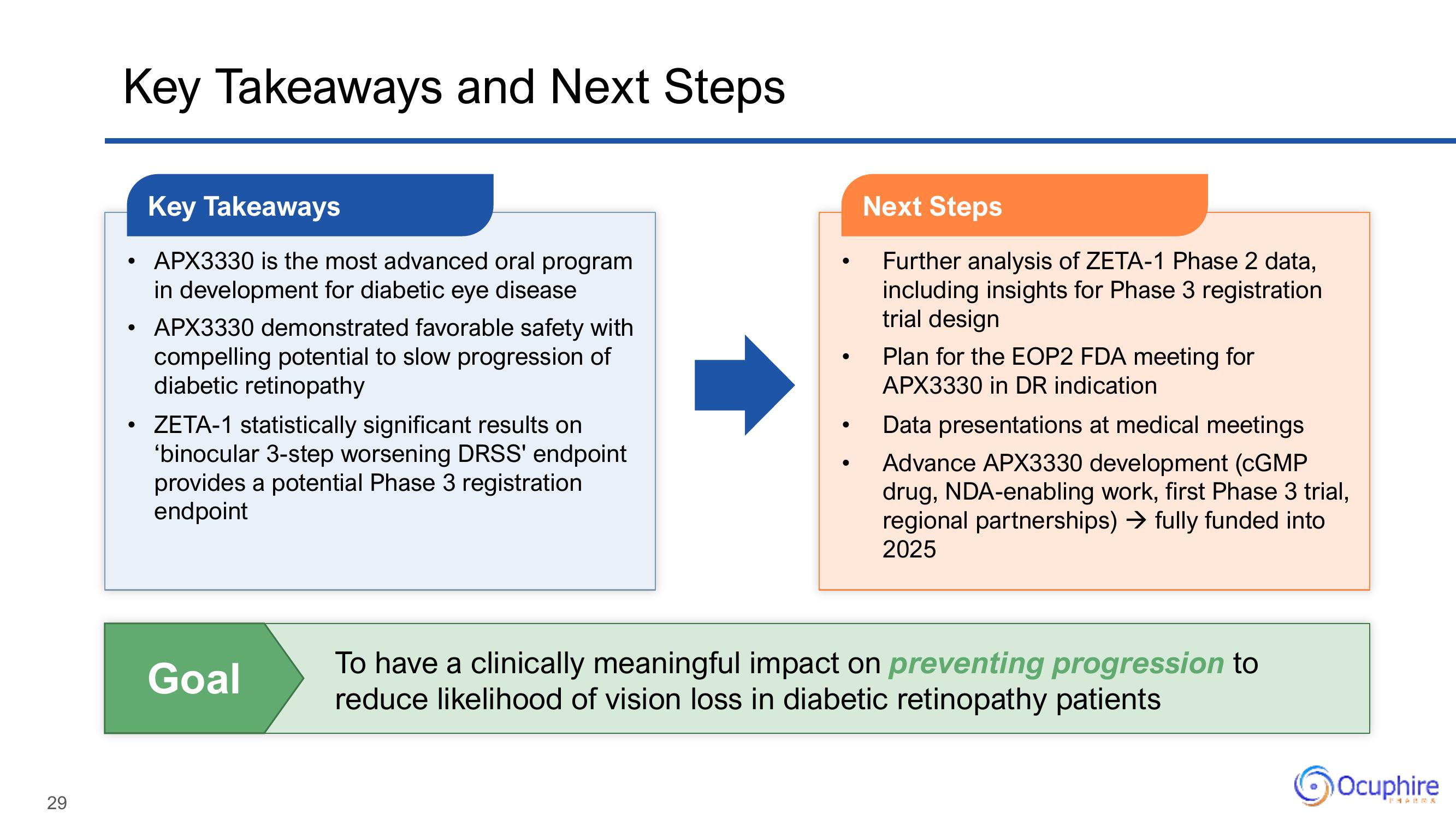
Key Takeaways and Next Steps
Key Takeaways
• APX3330 is the most advanced oral program
in development for diabetic eye disease
• APX3330 demonstrated favorable safety with
compelling potential to slow progression of
diabetic retinopathy
• ZETA-1 statistically significant results on
'binocular 3-step worsening DRSS' endpoint
provides a potential Phase 3 registration
endpoint
Goal
Next Steps
Further analysis of ZETA-1 Phase 2 data,
including insights for Phase 3 registration
trial design
Plan for the EOP2 FDA meeting for
APX3330 in DR indication
Data presentations at medical meetings
Advance APX3330 development (CGMP
drug, NDA-enabling work, first Phase 3 trial,
regional partnerships) → fully funded into
2025
To have a clinically meaningful impact on preventing progression to
reduce likelihood of vision loss in diabetic retinopathy patients
OcuphireView entire presentation