Calliditas Therapeutics IPO Presentation Deck
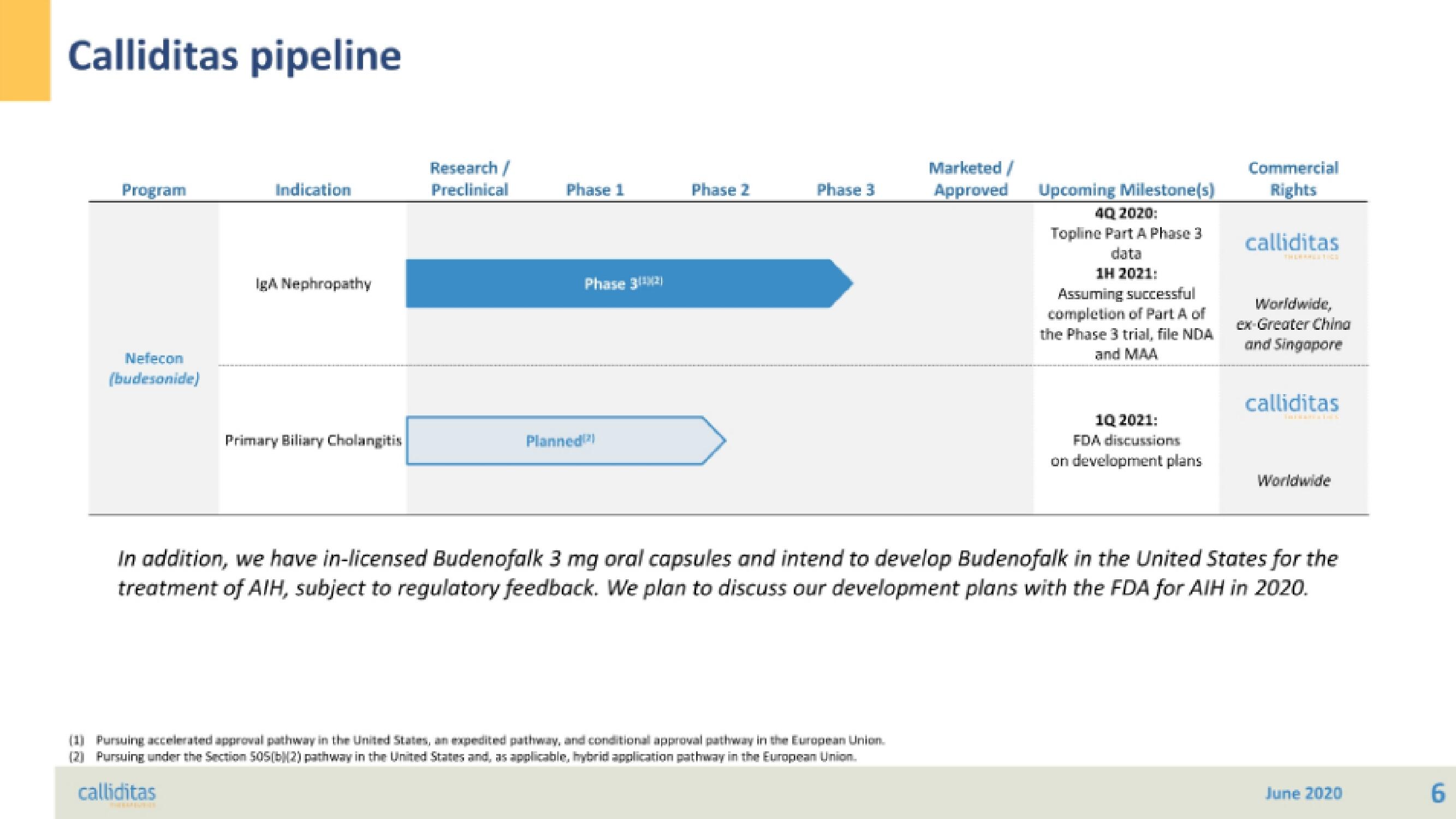
Calliditas pipeline
Program
Nefecon
(budesonide)
Indication
IgA Nephropathy
Primary Biliary Cholangitis
Research/
Preclinical
Phase 1
Planned
Phase 3(¹2)
Phase 2
Phase 3
Marketed/
Approved
(1) Pursuing accelerated approval pathway in the United States, an expedited pathway, and conditional approval pathway in the European Union.
(2) Pursuing under the Section 505(b)(2) pathway in the United States and, as applicable, hybrid application pathway in the European Union.
calliditas
Upcoming Milestone(s)
4Q 2020:
Topline Part A Phase 3
data
1H 2021:
Assuming successful
completion of Part A of
the Phase 3 trial, file NDA
and MAA
1Q 2021:
FDA discussions
on development plans
Commercial
Rights
calliditas
Worldwide,
ex-Greater China
and Singapore
calliditas
Worldwide
In addition, we have in-licensed Budenofalk 3 mg oral capsules and intend to develop Budenofalk in the United States for the
treatment of AIH, subject to regulatory feedback. We plan to discuss our development plans with the FDA for AIH in 2020.
June 2020
6View entire presentation