Valneva IPO Presentation Deck
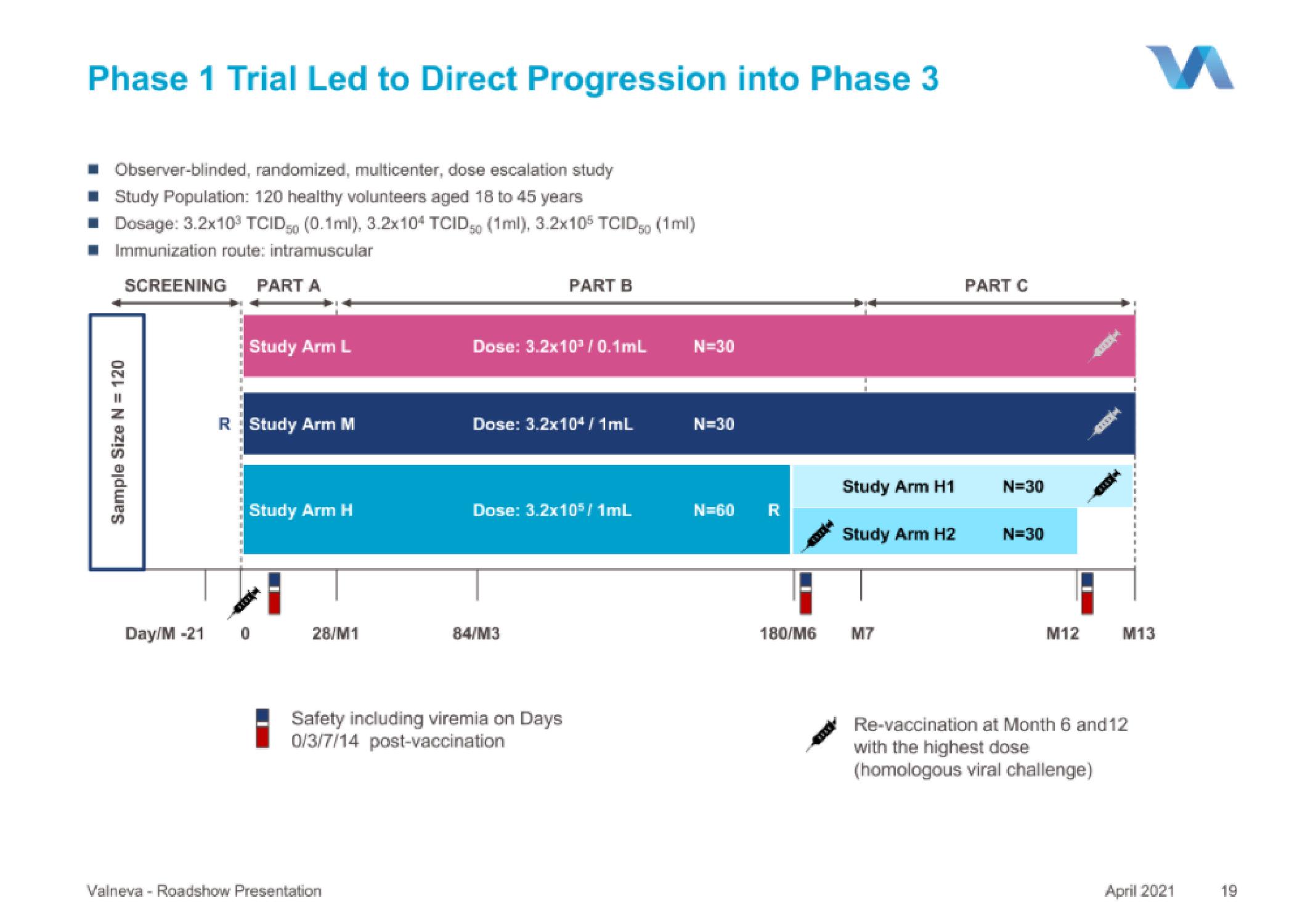
Phase 1 Trial Led to Direct Progression into Phase 3
■ Observer-blinded, randomized, multicenter, dose escalation study
■ Study Population: 120 healthy volunteers aged 18 to 45 years
50
■ Dosage: 3.2x10³ TCID 50 (0.1ml), 3.2x104 TCID (1ml), 3.2x105 TCID50 (1ml)
■ Immunization route: intramuscular
SCREENING PART A
Sample Size N = 120
Day/M -21
Study Arm L
R Study Arm M
H
Study Arm H
****
0
28/M1
Valneva - Roadshow Presentation
Dose: 3.2x10³/0.1mL
PART B
Dose: 3.2x104/1mL
Dose: 3.2x105 / 1mL
84/M3
Safety including viremia on Days
0/3/7/14 post-vaccination
N=30
N=30
N=60 R
RYT
Study Arm H1
Study Arm H2
180/M6 M7
*****
PART C
N=30
N=30
M12
D
COD-
****
V
M13
Re-vaccination at Month 6 and 12
with the highest dose
(homologous viral challenge)
April 2021
19View entire presentation