Neumora Therapeutics IPO Presentation Deck
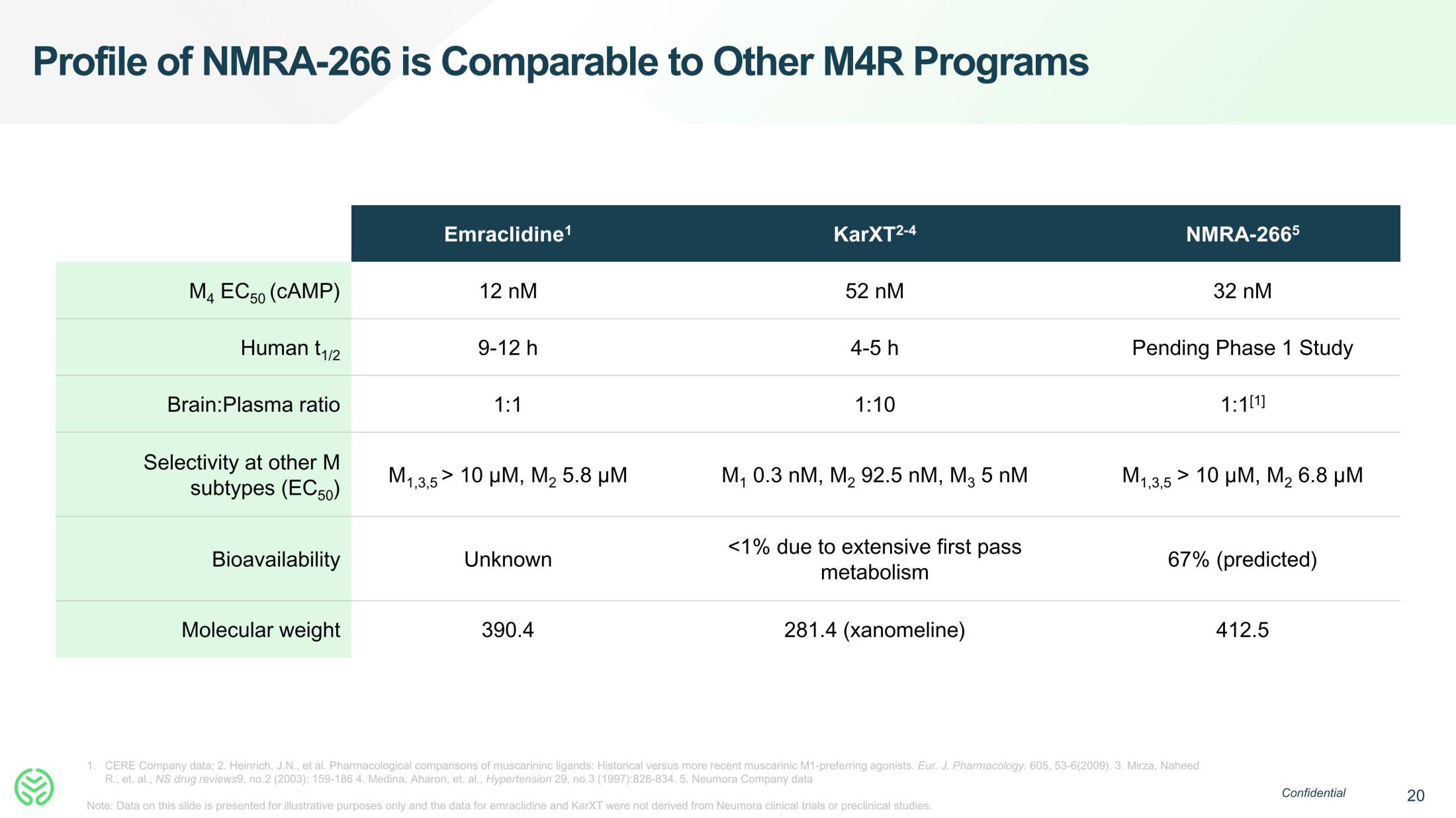
Profile of NMRA-266 is Comparable to Other M4R Programs
M4 EC50 (CAMP)
Human t₁/2
Brain:Plasma ratio
Selectivity at other M
subtypes (EC50)
Bioavailability
Molecular weight
Emraclidine¹
12 nM
M1,3,5>
9-12 h
1:1
;> 10 μΜ, Μ, 5.8 μΜ
Unknown
390.4
KarXT2-4
52 nM
4-5 h
1:10
M₁ 0.3 nM, M₂ 92.5 nM, M3 5 nM
<1% due to extensive first pass
metabolism
281.4 (xanomeline)
NMRA-2665
32 nM
Pending Phase 1 Study
1:1[1]
Μ1,3,5 > 10 μΜ, Μ, 6.8 μΜ
1. CERE Company data; 2. Heinrich, J.N., et al. Pharmacological comparisons of muscarininc ligands: Historical versus more recent muscarinic M1-preferring agonists. Eur. J. Pharmacology. 605, 53-6(2009). 3. Mirza, Naheed
R., et. al., NS drug reviews9, no.2 (2003): 159-186 4. Medina, Aharon, et. al., Hypertension 29, no.3 (1997):828-834. 5. Neumora Company data
Note: Data on this slide is presented for illustrative purposes only and the data for emraclidine and KarXT were not derived from Neumora clinical trials or preclinical studies.
67% (predicted)
412.5
Confidential
20View entire presentation