Immix Biopharma Investor Presentation Deck
Clinical Trial and Supply Agreement with BeiGene for Tislelizumab (Anti-PD-1 mAb)
●
IMMIX
ΒΙΟΡΗΑR ΜΑ
Study Goal
Demonstrate the potential for TSTX to be an integral
component of combination therapies for a wide range of
advanced solid tumors
Rapid IMX-110 expansion into additional oncology
indications
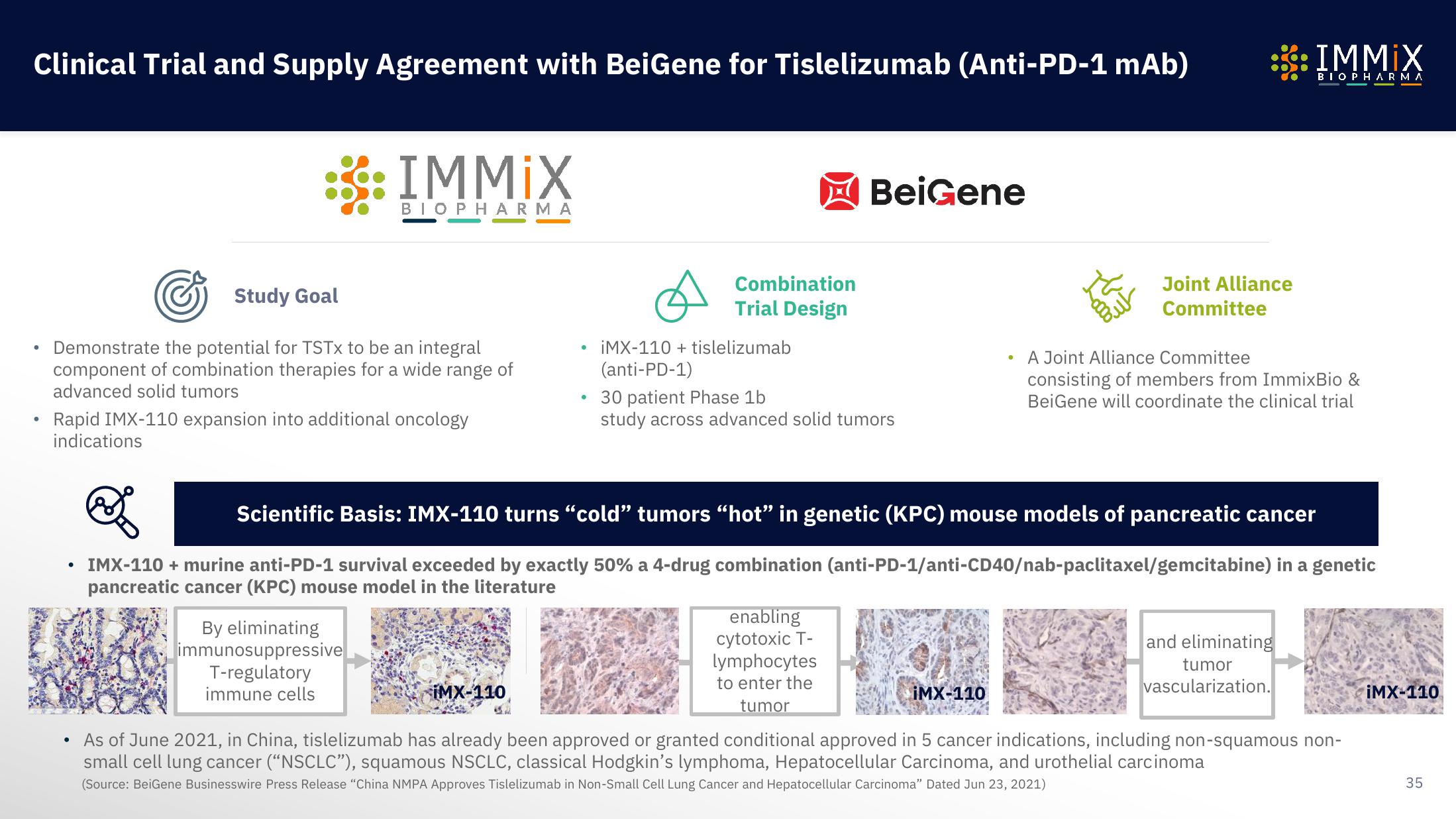
By eliminating
immunosuppressive
T-regulatory
immune cells
IMX-110
●
MAN
Combination
Trial Design
¡MX-110 + tislelizumab
(anti-PD-1)
BeiGene
30 patient Phase 1b
study across advanced solid tumors
enabling
cytotoxic T-
lymphocytes
to enter the
tumor
of
Scientific Basis: IMX-110 turns “cold” tumors “hot” in genetic (KPC) mouse models of pancreatic cancer
IMX-110 + murine anti-PD-1 survival exceeded by exactly 50% a 4-drug combination (anti-PD-1/anti-CD40/nab-paclitaxel/gemcitabine) in a genetic
pancreatic cancer (KPC) mouse model in the literature
●●●
S
¡MX-110
Joint Alliance
Committee
IMMIX
BIOPHARMA
A Joint Alliance Committee
consisting of members from ImmixBio &
BeiGene will coordinate the clinical trial
and eliminating
tumor
vascularization.
As of June 2021, in China, tislelizumab has already been approved or granted conditional approved in 5 cancer indications, including non-squamous non-
small cell lung cancer ("NSCLC"), squamous NSCLC, classical Hodgkin's lymphoma, Hepatocellular Carcinoma, and urothelial carcinoma
(Source: BeiGene Businesswire Press Release "China NMPA Approves Tislelizumab in Non-Small Cell Lung Cancer and Hepatocellular Carcinoma" Dated Jun 23, 2021)
iMX-110
35View entire presentation