BioAtla Investor Presentation Deck
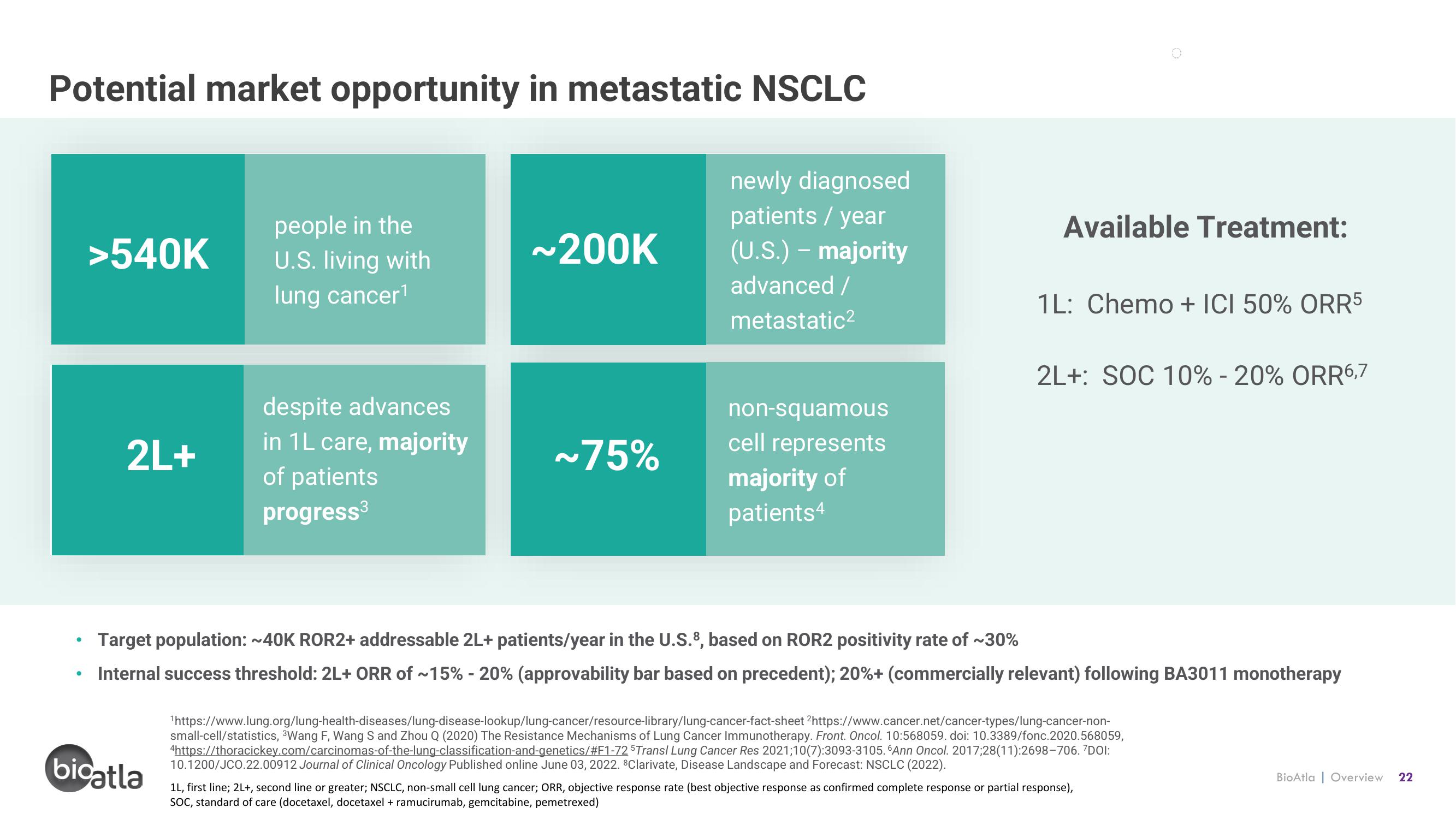
Potential market opportunity in metastatic NSCLC
●
>540K
2L+
people in the
U.S. living with
lung cancer¹
bicatla
despite advances
in 1L care, majority
of patients
progress³
~200K
~75%
newly diagnosed
patients / year
(U.S.) - majority
advanced /
metastatic²
non-squamous
cell represents
majority of
patients4
Available Treatment:
1L: Chemo + ICI 50% ORR5
2L+: SOC 10% - 20% ORR6,7
Target population: ~40K ROR2+ addressable 2L+ patients/year in the U.S.8, based on ROR2 positivity rate of ~30%
Internal success threshold: 2L+ ORR of ~15% - 20% (approvability bar based on precedent); 20%+ (commercially relevant) following BA3011 monotherapy
¹https://www.lung.org/lung-health-diseases/lung-disease-lookup/lung-cancer/resource-library/lung-cancer-fact-sheet 2https://www.cancer.net/cancer-types/lung-cancer-non-
small-cell/statistics, ³Wang F, Wang S and Zhou Q (2020) The Resistance Mechanisms of Lung Cancer Immunotherapy. Front. Oncol. 10:568059. doi: 10.3389/fonc.2020.568059,
4https://thoracickey.com/carcinomas-of-the-lung-classification-and-genetics/#F1-72 5Transl Lung Cancer Res 2021;10(7):3093-3105. Ann Oncol. 2017;28(11):2698-706. DOI:
10.1200/JCO.22.00912 Journal of Clinical Oncology Published online June 03, 2022. Clarivate, Disease Landscape and Forecast: NSCLC (2022).
1L, first line; 2L+, second line or greater; NSCLC, non-small cell lung cancer; ORR, objective response rate (best objective response as confirmed complete response or partial response),
SOC, standard of care (docetaxel, docetaxel + ramucirumab, gemcitabine, pemetrexed)
BioAtla| Overview 22View entire presentation