ATAI Investor Presentation Deck
SUMMARY
OWNERSHIP 58.9%¹
PRODUCT
PHARMA-
COLOGY
PRODUCT
FEATURES
INDICATIONS
CURRENT
STATUS
INTELLECTUAL
PROPERTY
HIGHLIGHT
Subcutaneous R-ketamine (PCN-101)
Glutamatergic modulator
Rapid-acting, nonpsychedelic antidepressant
with potential for at home use
Primary: Treatment Resistant Depression
Potential: Substance Use Disorder
Phase 1 trial showed safety and tolerability
of R-ketamine at doses of up to 150mg,
Phase 2a proof-of-concept study initiated
in Q3 2021
Issued methods of use of R-ketamine for
treatment of depressive symptoms
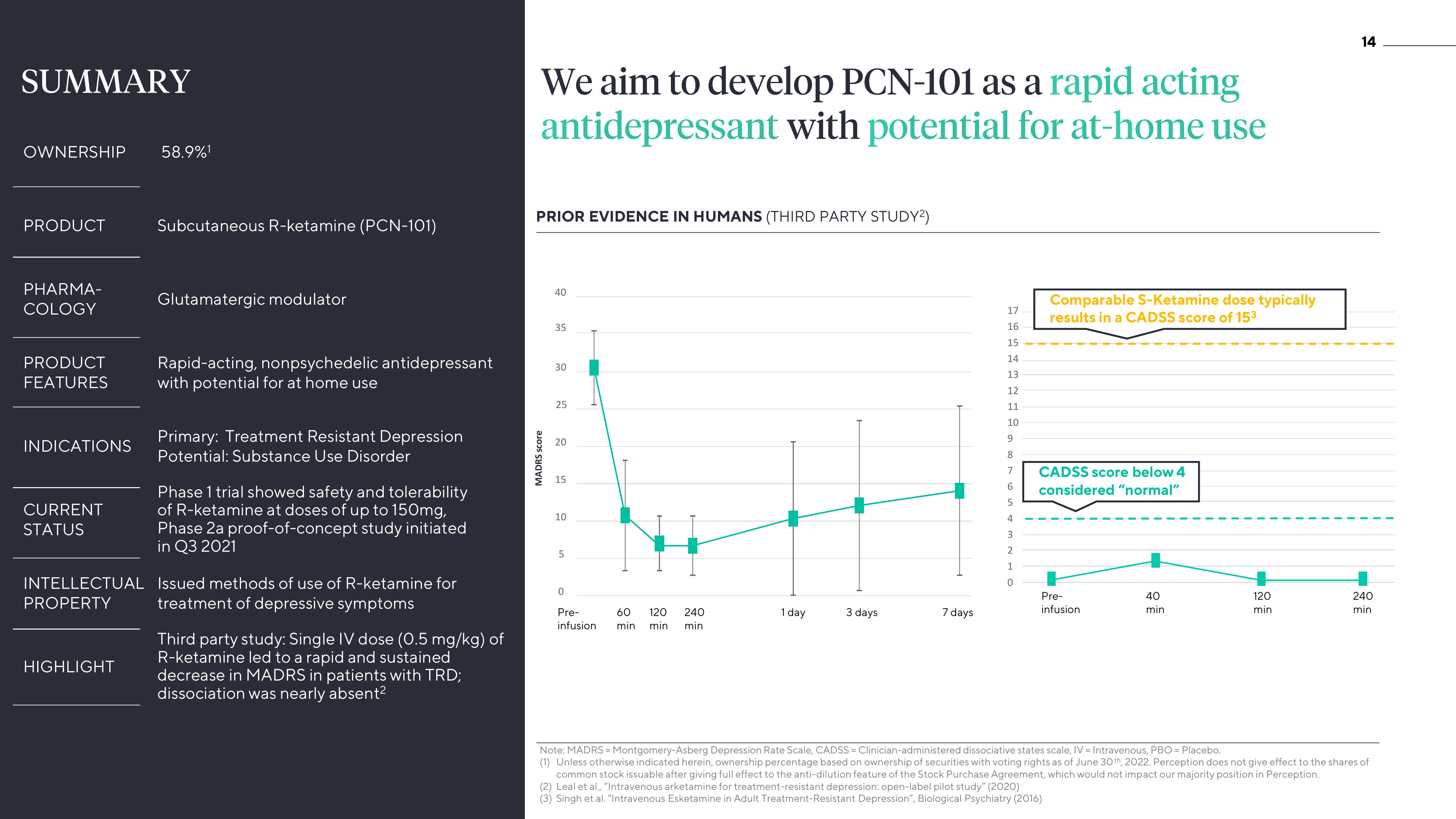
Third party study: Single IV dose (0.5 mg/kg) of
R-ketamine led to a rapid and sustained
decrease in MADRS in patients with TRD;
dissociation was nearly absent²
We aim to develop PCN-101 as a rapid acting
antidepressant with potential for at-home use
PRIOR EVIDENCE IN HUMANS (THIRD PARTY STUDY²)
MADRS score
40
35
30
25
20
15
10
5
0
Pre-
infusion
120 240
60
min min min
1 day
3 days
7 days
DGHHBERG
17
16
15
14
13
12
11
10
9
8
7
6
5
4
3
2
1
0
Comparable S-Ketamine dose typically
results in a CADSS score of 153
CADSS score below 4
considered "normal"
Pre-
infusion
40
min
120
min
14
240
min
Note: MADRS = Montgomery-Asberg Depression Rate Scale, CADSS=Clinician-administered dissociative states scale, IV = Intravenous, PBO = Placebo.
(1) Unless otherwise indicated herein, ownership percentage based on ownership of securities with voting rights as of June 30th, 2022. Perception does not give effect to the shares of
common stock issuable after giving full effect to the anti-dilution feature of the Stock Purchase Agreement, which would not impact our majority position in Perception.
(2) Leal et al., "Intravenous arketamine for treatment-resistant depression: open-label pilot study" (2020)
(3) Singh et al. "Intravenous Esketamine in Adult Treatment-Resistant Depression", Biological Psychiatry (2016)View entire presentation