Kymera Investor Presentation Deck
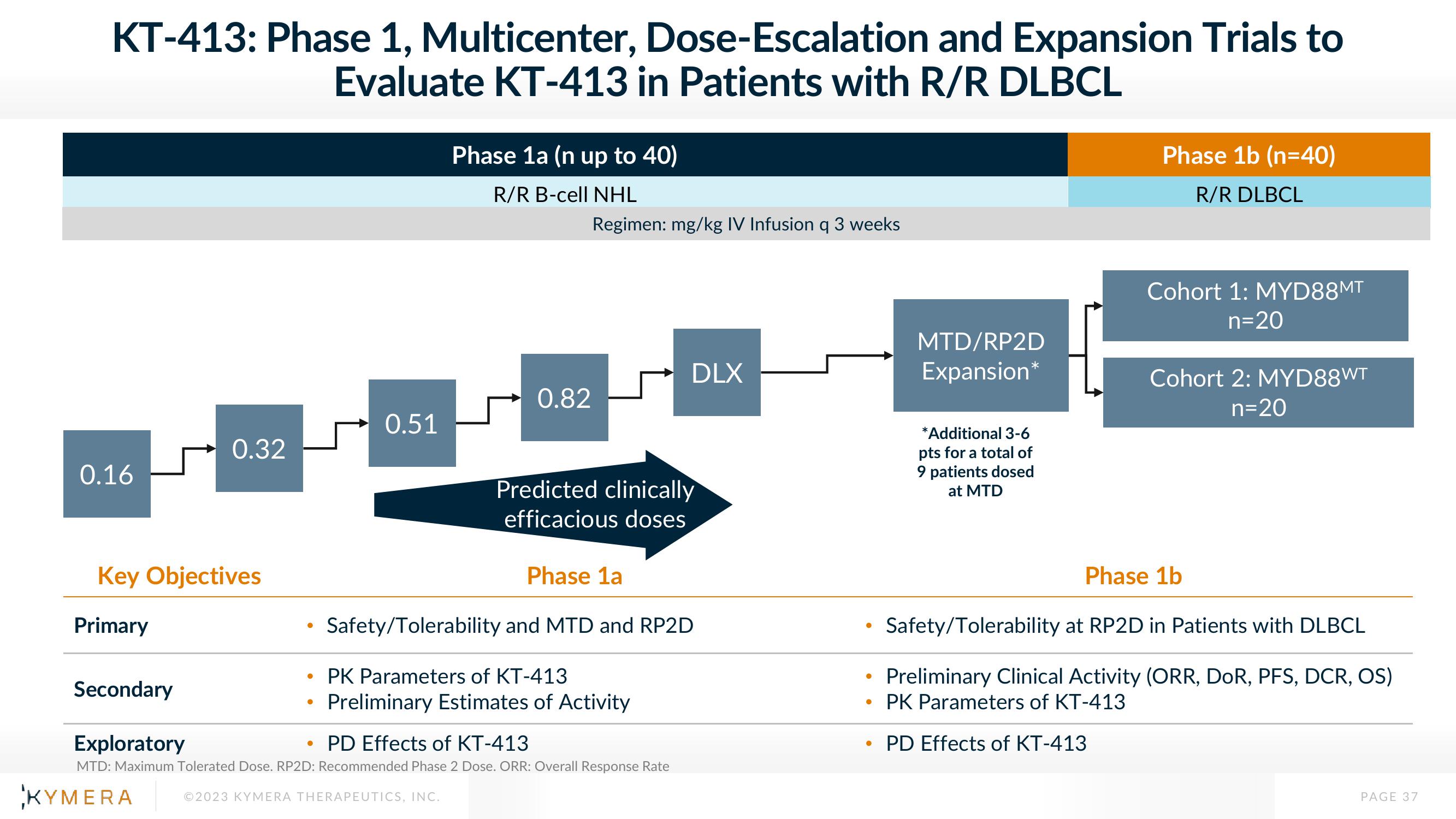
KT-413: Phase 1, Multicenter, Dose-Escalation and Expansion Trials to
Evaluate KT-413 in Patients with R/R DLBCL
0.16
Key Objectives
Primary
0.32
Secondary
●
Ⓡ
0.51
●
Phase 1a (n up to 40)
R/R B-cell NHL
0.82
Regimen: mg/kg IV Infusion q 3 weeks
Predicted clinically
efficacious doses
DLX
Phase 1a
Safety/Tolerability and MTD and RP2D
PK Parameters of KT-413
Preliminary Estimates of Activity
Exploratory
PD Effects of KT-413
MTD: Maximum Tolerated Dose. RP2D: Recommended Phase 2 Dose. ORR: Overall Response Rate
KYMERA ©2023 KYMERA THERAPEUTICS, INC.
●
●
●
MTD/RP2D
Expansion
*
*Additional 3-6
pts for a total of
9 patients dosed
at MTD
Phase 1b (n=40)
R/R DLBCL
PD Effects of KT-413
Cohort 1: MYD88MT
n=20
Cohort 2: MYD88WT
n=20
Phase 1b
Safety/Tolerability at RP2D in Patients with DLBCL
Preliminary Clinical Activity (ORR, DOR, PFS, DCR, OS)
PK Parameters of KT-413
PAGE 37View entire presentation