Centessa IPO Presentation Deck
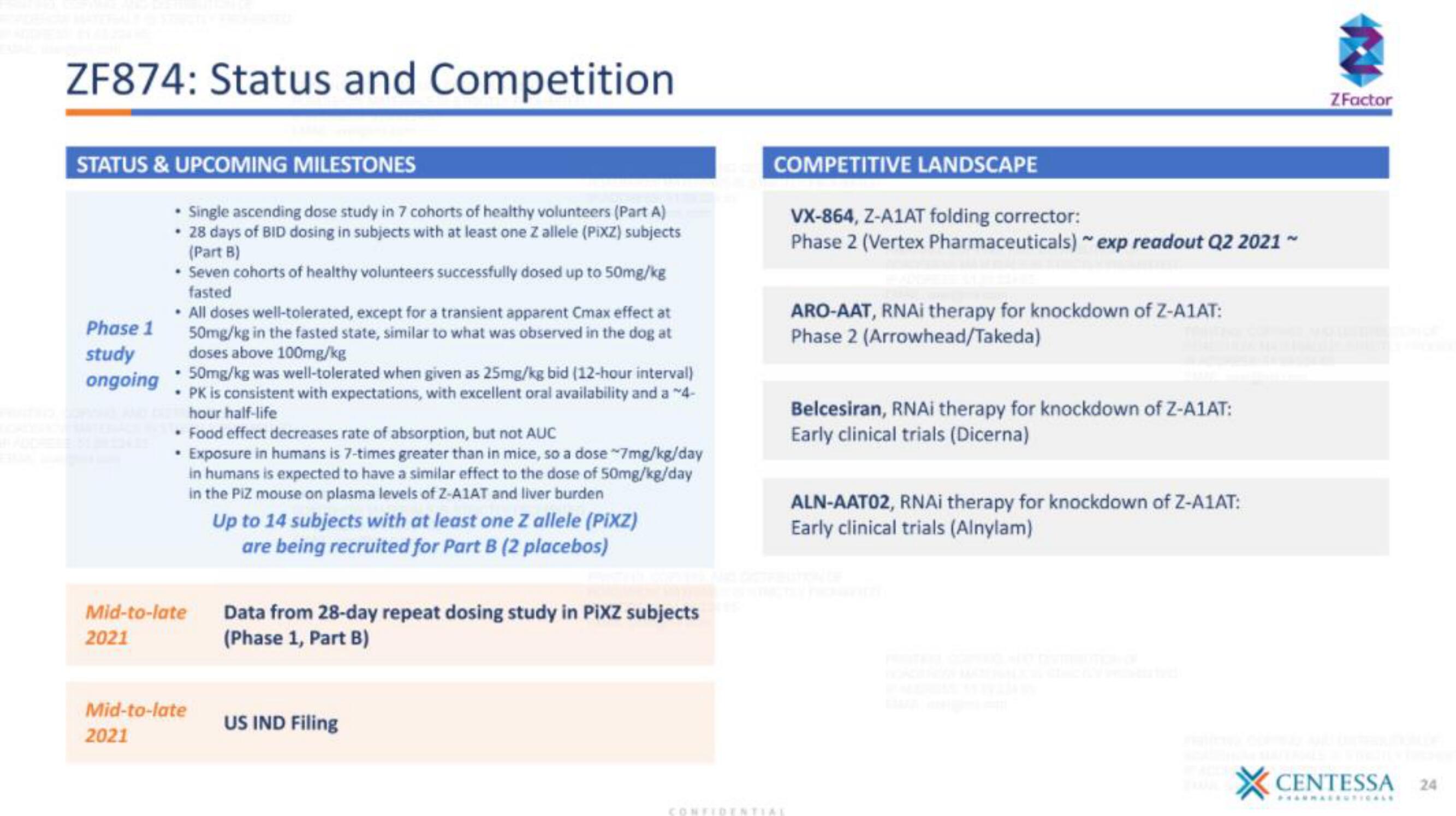
ZF874: Status and Competition
STATUS & UPCOMING MILESTONES
Phase 1
study
ongoing
• Single ascending dose study in 7 cohorts of healthy volunteers (Part A)
• 28 days of BID dosing in subjects with at least one Z allele (PiXZ) subjects
(Part B)
• Seven cohorts of healthy volunteers successfully dosed up to 50mg/kg
fasted
• All doses well-tolerated, except for a transient apparent Cmax effect at
50mg/kg in the fasted state, similar to what was observed in the dog at
doses above 100mg/kg
• 50mg/kg was well-tolerated when given as 25mg/kg bid (12-hour interval)
• PK is consistent with expectations, with excellent oral availability and a ~4-
hour half-life
• Food effect decreases rate of absorption, but not AUC
• Exposure in humans is 7-times greater than in mice, so a dose ~7mg/kg/day
Mid-to-late
2021
Mid-to-late
2021
in humans is expected to have a similar effect to the dose of 50mg/kg/day
in the PIZ mouse on plasma levels of Z-A1AT and liver burden
Up to 14 subjects with at least one Z allele (PiXZ)
are being recruited for Part B (2 placebos)
Data from 28-day repeat dosing study in PiXZ subjects
(Phase 1, Part B)
US IND Filing
COMPETITIVE LANDSCAPE
VX-864, Z-A1AT folding corrector:
Phase 2 (Vertex Pharmaceuticals) ~ exp readout Q2 2021 ~
ARO-AAT, RNAi therapy for knockdown of Z-A1AT:
Phase 2 (Arrowhead/Takeda)
Belcesiran, RNAi therapy for knockdown of Z-A1AT:
Early clinical trials (Dicerna)
ALN-AATO2, RNAi therapy for knockdown of Z-A1AT:
Early clinical trials (Alnylam)
ZFactor
CENTESSA
24View entire presentation