Equillium Results Presentation Deck
Approved Therapies: Need Remains for Rapid & Deep Responses
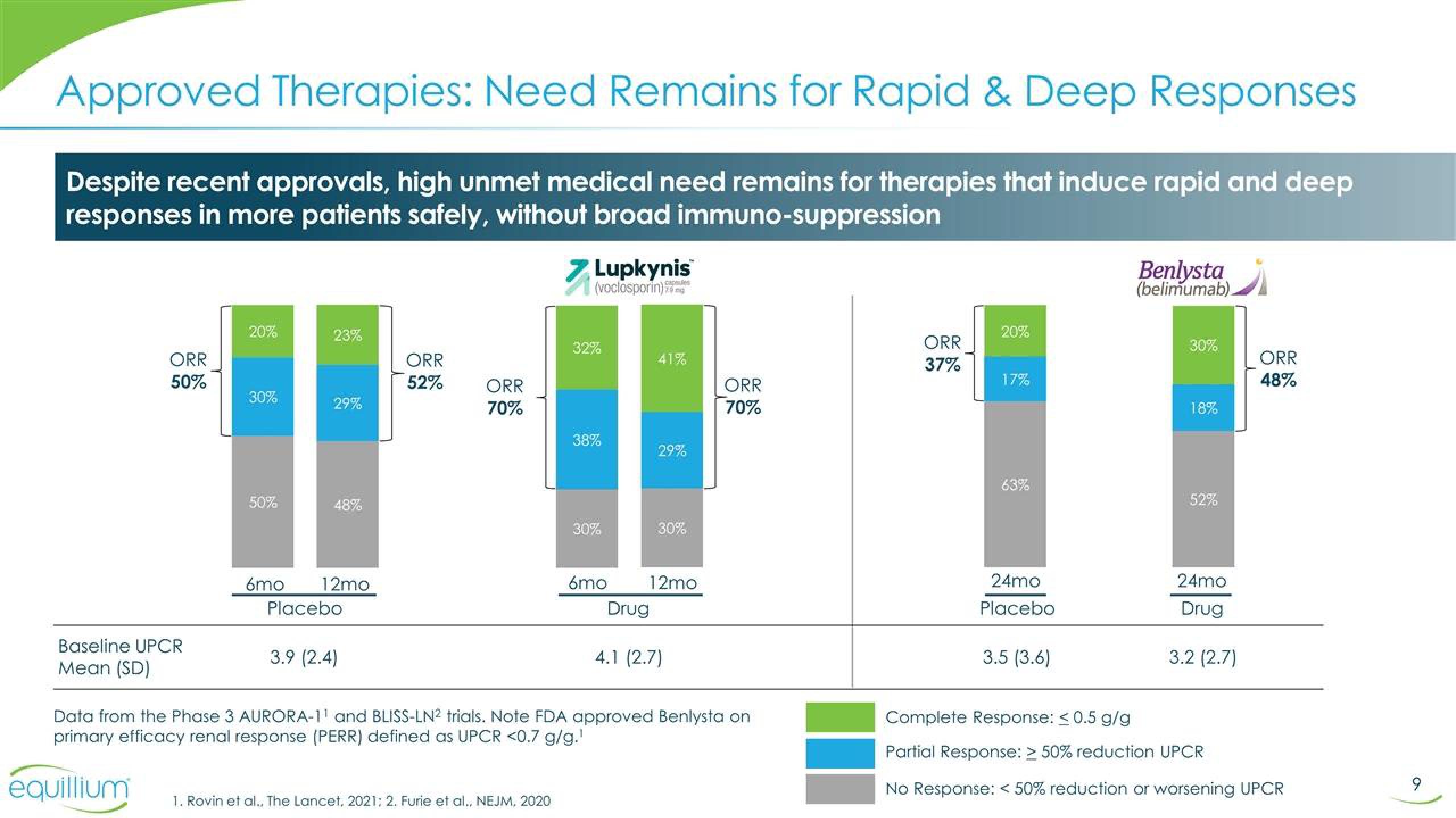
Despite recent approvals, high unmet medical need remains for therapies that induce rapid and deep
responses in more patients safely, without broad immuno-suppression
ORR
50%
Baseline UPCR
Mean (SD)
20%
30%
50%
23%
29%
48%
6mo 12mo
Placebo
3.9 (2.4)
ORR
52%
ORR
70%
> Lupkynis
(voclosporin)
1. Rovin et al., The Lancet, 2021; 2. Furie et al., NEJM, 2020
32%
38%
30%
6mo
Drug
capsules
41%
29%
30%
12mo
4.1 (2.7)
ORR
70%
Data from the Phase 3 AURORA-1¹ and BLISS-LN2 trials. Note FDA approved Benlysta on
primary efficacy renal response (PERR) defined as UPCR <0.7 g/g.¹
equillium
ORR
37%
20%
17%
63%
24mo
Placebo
3.5 (3.6)
Benlysta
(belimumab)
30%
18%
52%
24mo
Drug
3.2 (2.7)
ORR
48%
Complete Response: < 0.5 g/g
Partial Response: > 50% reduction UPCR
No Response: < 50% reduction or worsening UPCR
aView entire presentation