Ocuphire Pharma Investor Day Presentation Deck
RM
58
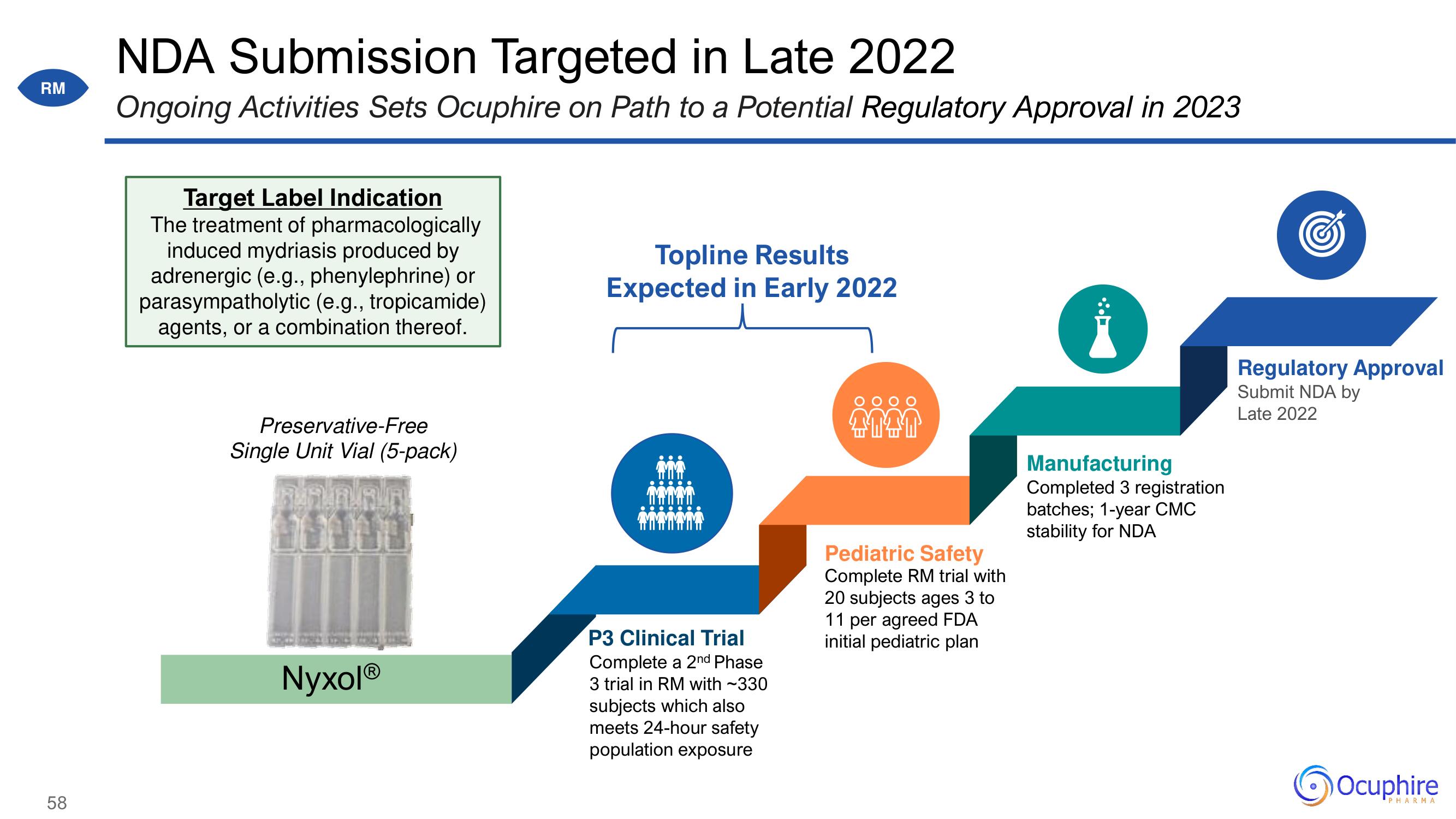
NDA Submission Targeted in Late 2022
Ongoing Activities Sets Ocuphire on Path to a Potential Regulatory Approval in 2023
Target Label Indication
The treatment of pharmacologically
induced mydriasis produced by
adrenergic (e.g., phenylephrine) or
parasympatholytic (e.g., tropicamide)
agents, or a combination thereof.
Preservative-Free
Single Unit Vial (5-pack)
NyxolⓇ
Topline Results
Expected in Early 2022
P3 Clinical Trial
Complete a 2nd Phase
3 trial in RM with ~330
subjects which also
meets 24-hour safety
population exposure
4141
Pediatric Safety
Complete RM trial with
20 subjects ages 3 to
11 per agreed FDA
initial pediatric plan
:
Manufacturing
Completed 3 registration
batches; 1-year CMC
stability for NDA
Regulatory Approval
Submit NDA by
Late 2022
Ocuphire
PHARMAView entire presentation