Context Therapeutics Investor Presentation Deck
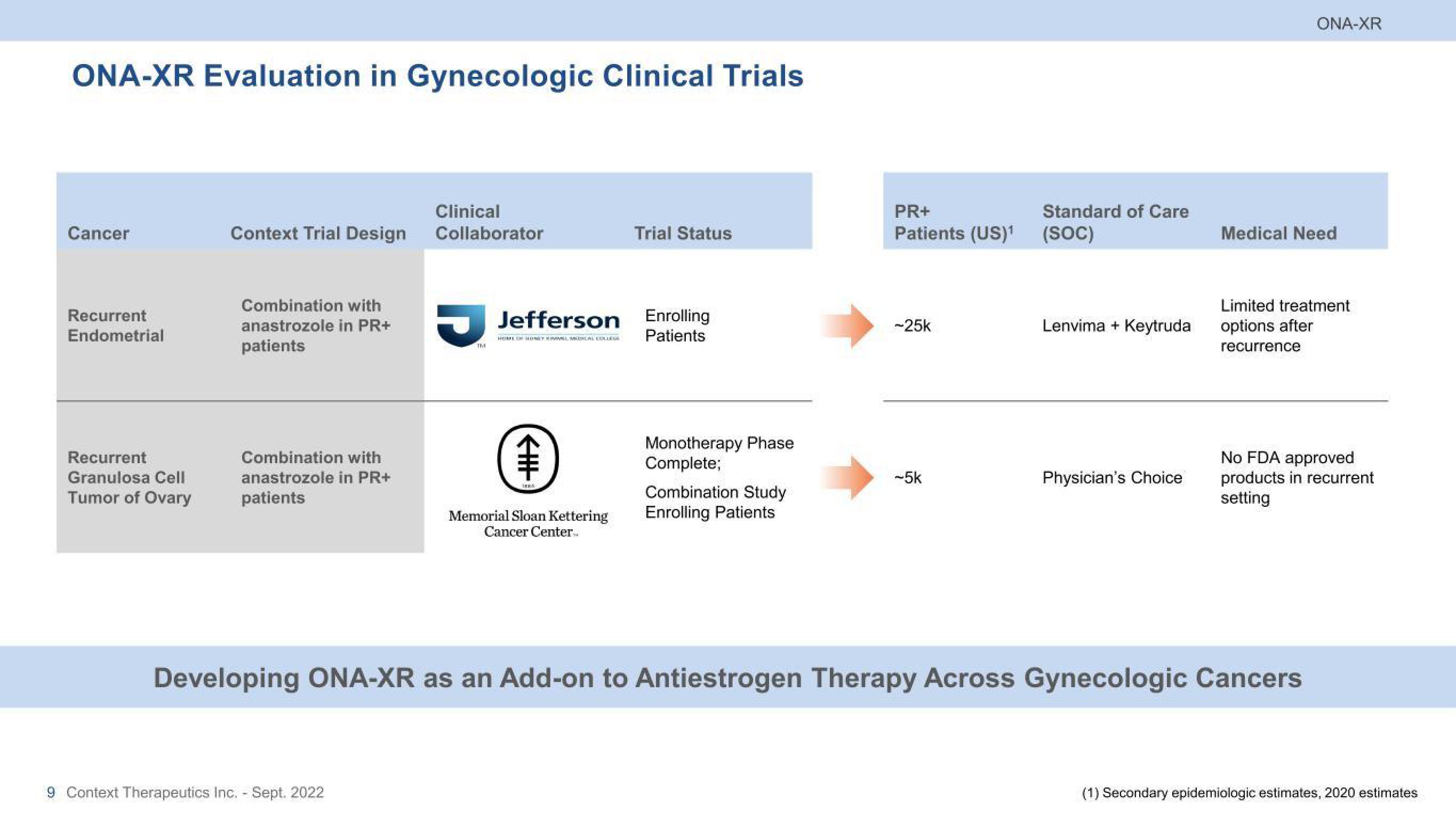
ONA-XR Evaluation in Gynecologic Clinical Trials
Cancer
Recurrent
Endometrial
Recurrent
Granulosa Cell
Tumor of Ovary
Context Trial Design
Combination with
anastrozole in PR+
patients
Combination with
anastrozole in PR+
patients
Clinical
Collaborator
9 Context Therapeutics Inc. - Sept. 2022
S
Jefferson
HOME HOMETRIIVEL MEDICAL COLLEGE
←tt
#
INKA
Memorial Sloan Kettering
Cancer Center...
Trial Status
Enrolling
Patients
Monotherapy Phase
Complete;
Combination Study
Enrolling Patients
PR+
Patients (US)¹ (SOC)
-25k
Standard of Care
-5k
Lenvima + Keytruda
Physician's Choice
ONA-XR
Medical Need
Limited treatment
options after
recurrence
Developing ONA-XR as an Add-on to Antiestrogen Therapy Across Gynecologic Cancers
No FDA approved
products in recurrent
setting
(1) Secondary epidemiologic estimates, 2020 estimatesView entire presentation