AstraZeneca Results Presentation Deck
Koselugo and Orpathys
'Rare' opportunities to make a significant difference in the lives of patients
Koselugo approved in the EU for children with neuro-
fibromatosis type 1 and plexiform neurofibromas (PN)
66%
Patients met the primary endpoint of ≥20%
reduction in target NF1 PN volume¹
82%
Patients with partial responses remained
in response after 12 months²
27%
Median best percentage change in
target NF1 PN volume from baseline
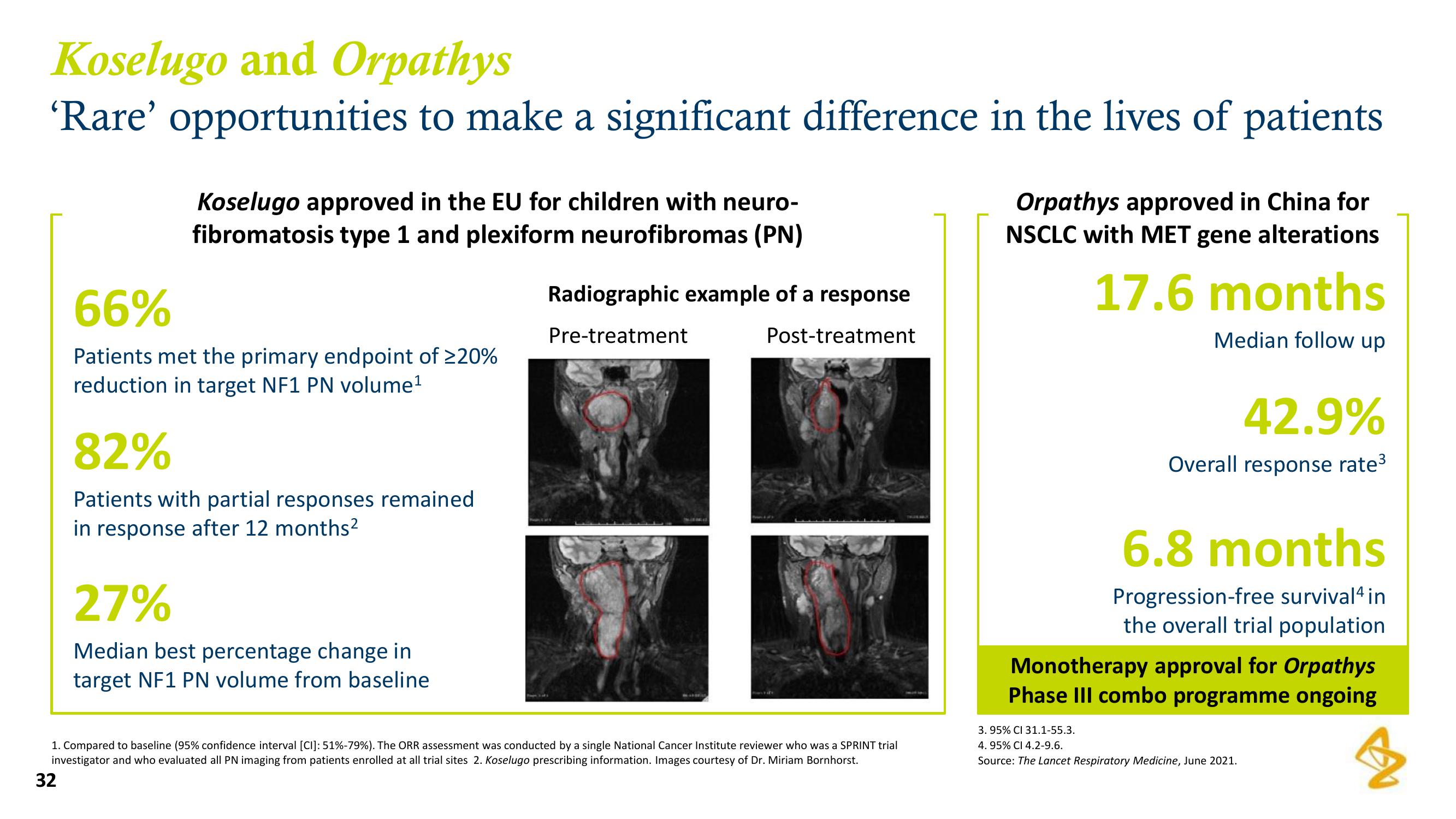
Radiographic example of a response
Pre-treatment
Post-treatment
1. Compared to baseline (95% confidence interval [CI]: 51%-79%). The ORR assessment was conducted by a single National Cancer Institute reviewer who was a SPRINT trial
investigator and who evaluated all PN imaging from patients enrolled at all trial sites Koselugo prescribing information. Images courtesy of Dr. Miriam Bornhorst.
32
Orpathys approved in China for
NSCLC with MET gene alterations
17.6 months
Median follow up
42.9%
Overall response rate³
6.8 months
Progression-free survival4 in
the overall trial population
Monotherapy approval for Orpathys
Phase III combo programme ongoing
3
3. 95% CI 31.1-55.3.
4. 95% CI 4.2-9.6.
Source: The Lancet Respiratory Medicine, June 2021.View entire presentation