BioAtla IPO Presentation Deck
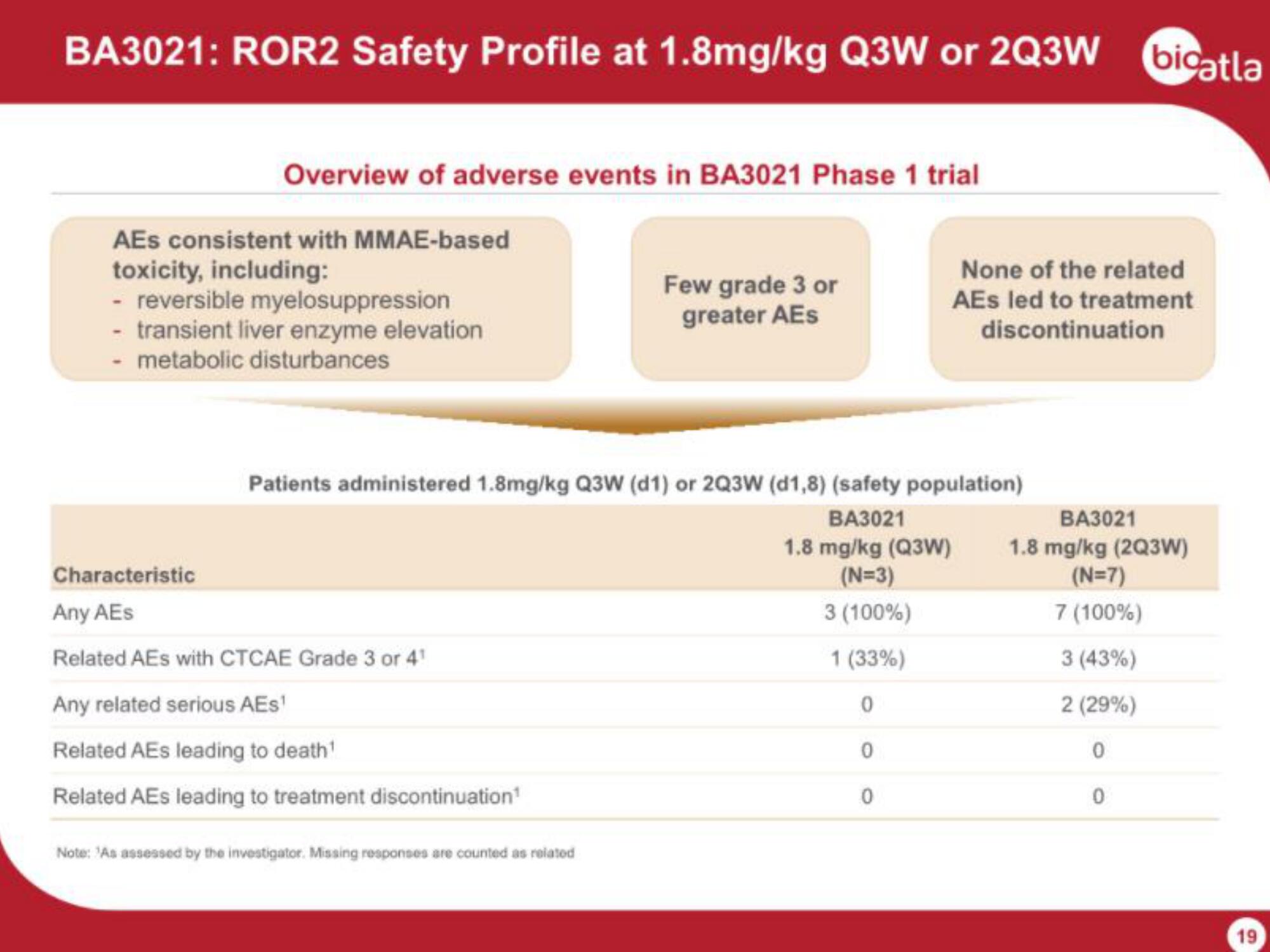
BA3021: ROR2 Safety Profile at 1.8mg/kg Q3W or 2Q3W bigatla
Overview of adverse events in BA3021 Phase 1 trial
AEs consistent with MMAE-based
toxicity, including:
- reversible myelosuppression
- transient liver enzyme elevation
- metabolic disturbances
Characteristic
Any AEs
Related AES with CTCAE Grade 3 or 4¹
Any related serious AEs¹
Related AEs leading to death¹
Related AEs leading to treatment discontinuation¹
Few grade 3 or
greater AES
Patients administered 1.8mg/kg Q3W (d1) or 2Q3W (d1,8) (safety population)
BA3021
1.8 mg/kg (Q3W)
(N=3)
3 (100%)
1 (33%)
Note: "As assessed by the investigator. Missing responses are counted as related
0
0
None of the related
AEs led to treatment
discontinuation
0
BA3021
1.8 mg/kg (2Q3W)
(N=7)
7 (100%)
3 (43%)
2 (29%)
0
0
19View entire presentation