Eleusis SPAC
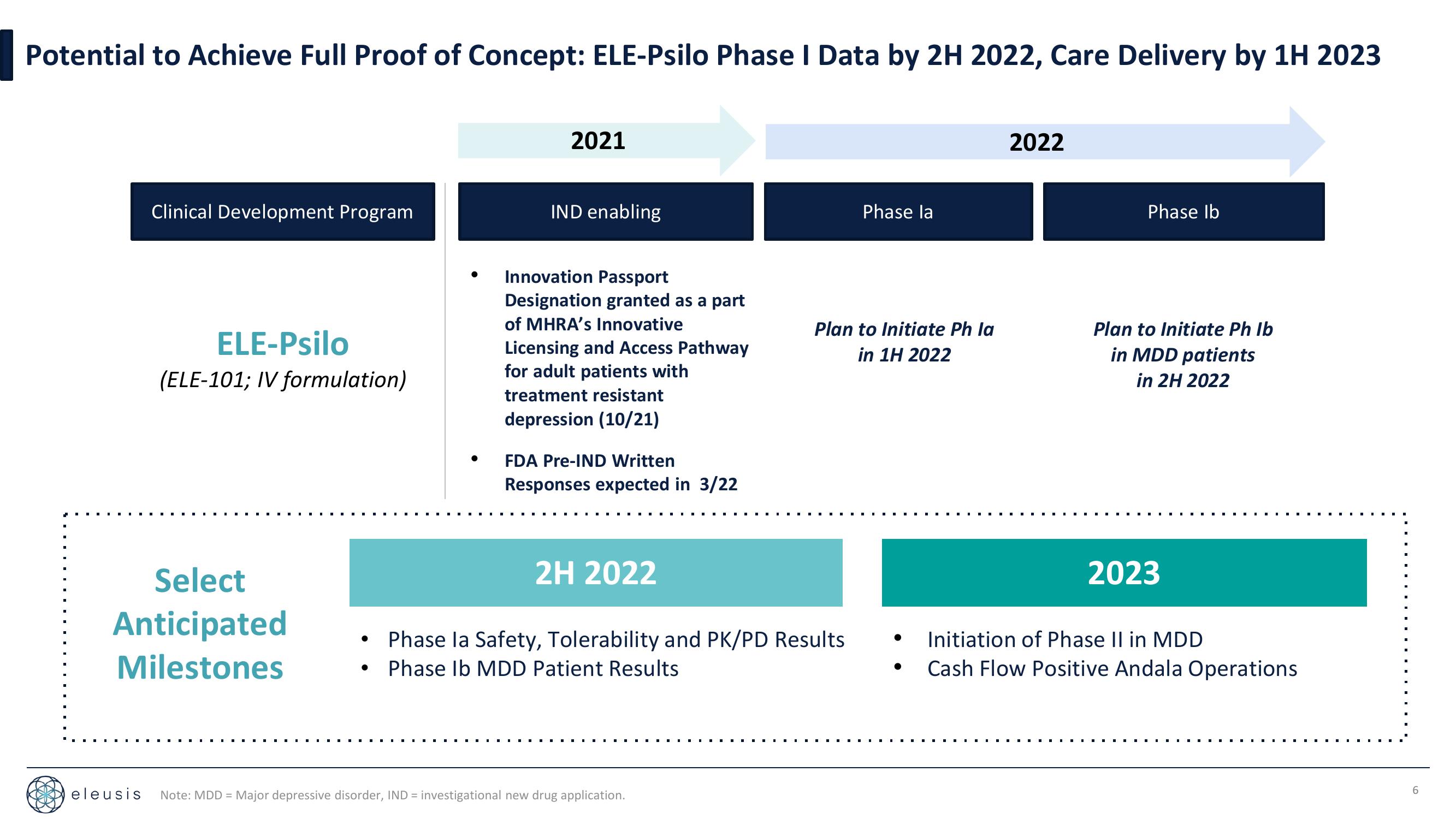
Potential to Achieve Full Proof of Concept: ELE-Psilo Phase I Data by 2H 2022, Care Delivery by 1H 2023
Clinical Development Program
ELE-Psilo
(ELE-101; IV formulation)
Select
Anticipated
Milestones
●
2021
IND enabling
Innovation Passport
Designation granted as a part
of MHRA's Innovative
Licensing and Access Pathway
for adult patients with
treatment resistant
depression (10/21)
FDA Pre-IND Written
Responses expected in 3/22
2H 2022
Phase la Safety, Tolerability and PK/PD Results
Phase Ib MDD Patient Results
eleusis Note: MDD = Major depressive disorder, IND = investigational new drug application.
Phase la
Plan to Initiate Ph la
in 1H 2022
●
2022
Phase Ib
Plan to Initiate Ph Ib
in MDD patients
in 2H 2022
2023
Initiation of Phase II in MDD
Cash Flow Positive Andala Operations
6View entire presentation