Imara M&A
Evolving Chronic Myeloid Leukemia Market Dynamics
Current Market
Growing patient population due to improved survival, requiring some
patients to be on TKIs for decades
Existing approved drugs have liabilities as chronic therapy, including
poor tolerability due to off-target effects and inability to dose to
maximal efficacy resulting in high switch rates
Nevertheless, the five approved drugs (including generics) drive
annual sales of >$6B, with every drug achieving ~$500M in sales and
multiple drugs achieving sales of >$2B
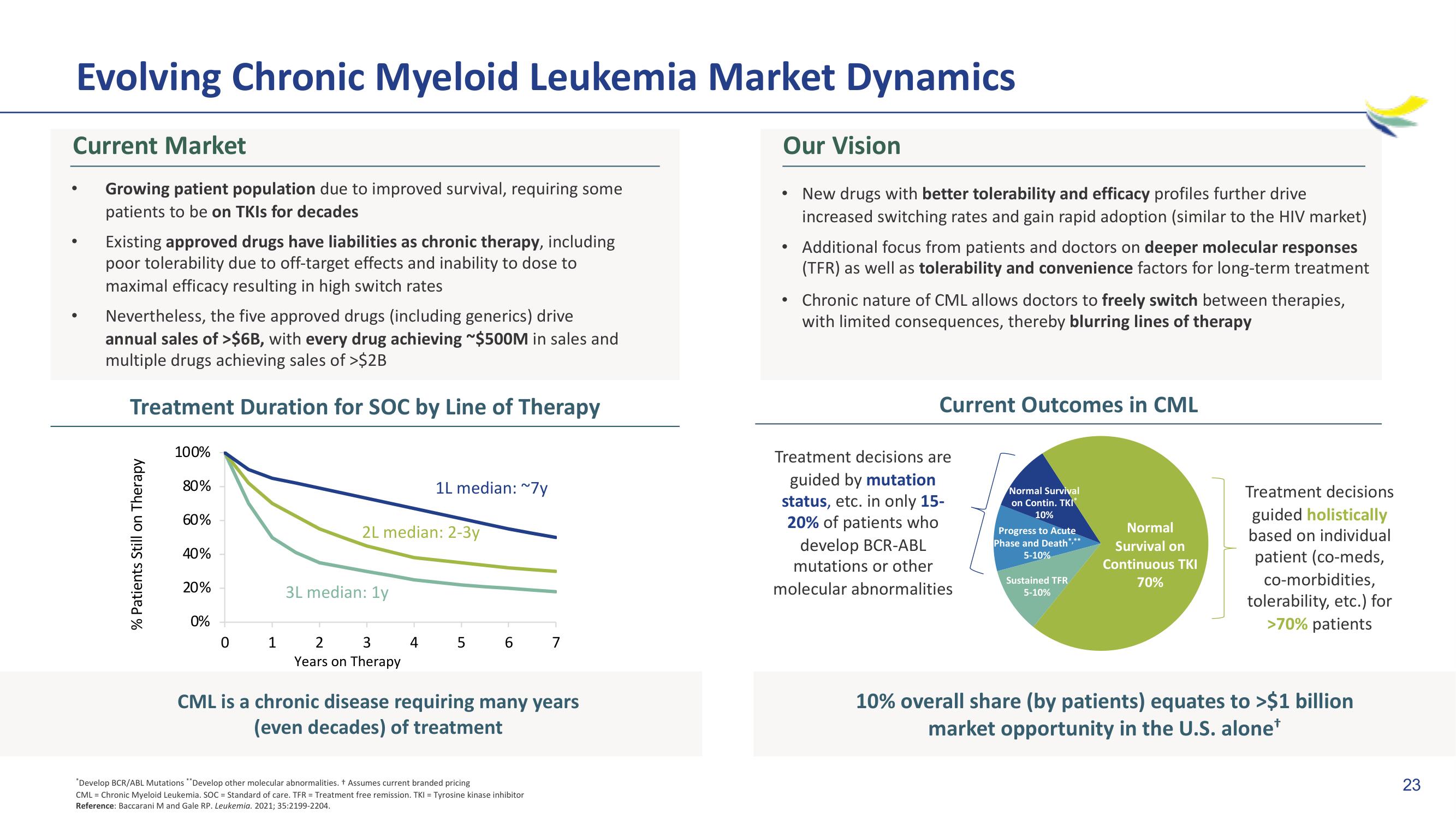
Treatment Duration for SOC by Line of Therapy
% Patients Still on Therapy
100%
80%
60%
40%
20%
0%
0
1
2L median: 2-3y
3L median: 1y
2 3
Years on Therapy
1L median: ~7y
4
5
6
7
CML is a chronic disease requiring many years
(even decades) of treatment
*Develop BCR/ABL Mutations "Develop other molecular abnormalities. † Assumes current branded pricing
CML = Chronic Myeloid Leukemia. SOC Standard of care. TFR = Treatment free remission. TKI = Tyrosine kinase inhibitor
Reference: Baccarani M and Gale RP. Leukemia. 2021; 35:2199-2204.
Our Vision
New drugs with better tolerability and efficacy profiles further drive
increased switching rates and gain rapid adoption (similar to the HIV market)
• Additional focus from patients and doctors on deeper molecular responses
(TFR) as well as tolerability and convenience factors for long-term treatment
Chronic nature of CML allows doctors to freely switch between therapies,
with limited consequences, thereby blurring lines of therapy
Current Outcomes in CML
Treatment decisions are
guided by mutation
status, etc. in only 15-
20% of patients who
develop BCR-ABL
mutations or other
molecular abnormalities
Normal Survival
on Contin. TKI
10%
Progress to Acute
Phase and Death***
5-10%
Sustained TFR
5-10%
Normal
Survival on
Continuous TKI
70%
Treatment decisions
guided holistically
based on individual
patient (co-meds,
co-morbidities,
tolerability, etc.) for
>70% patients
10% overall share (by patients) equates to >$1 billion
market opportunity in the U.S. alone*
23View entire presentation