Imara M&A
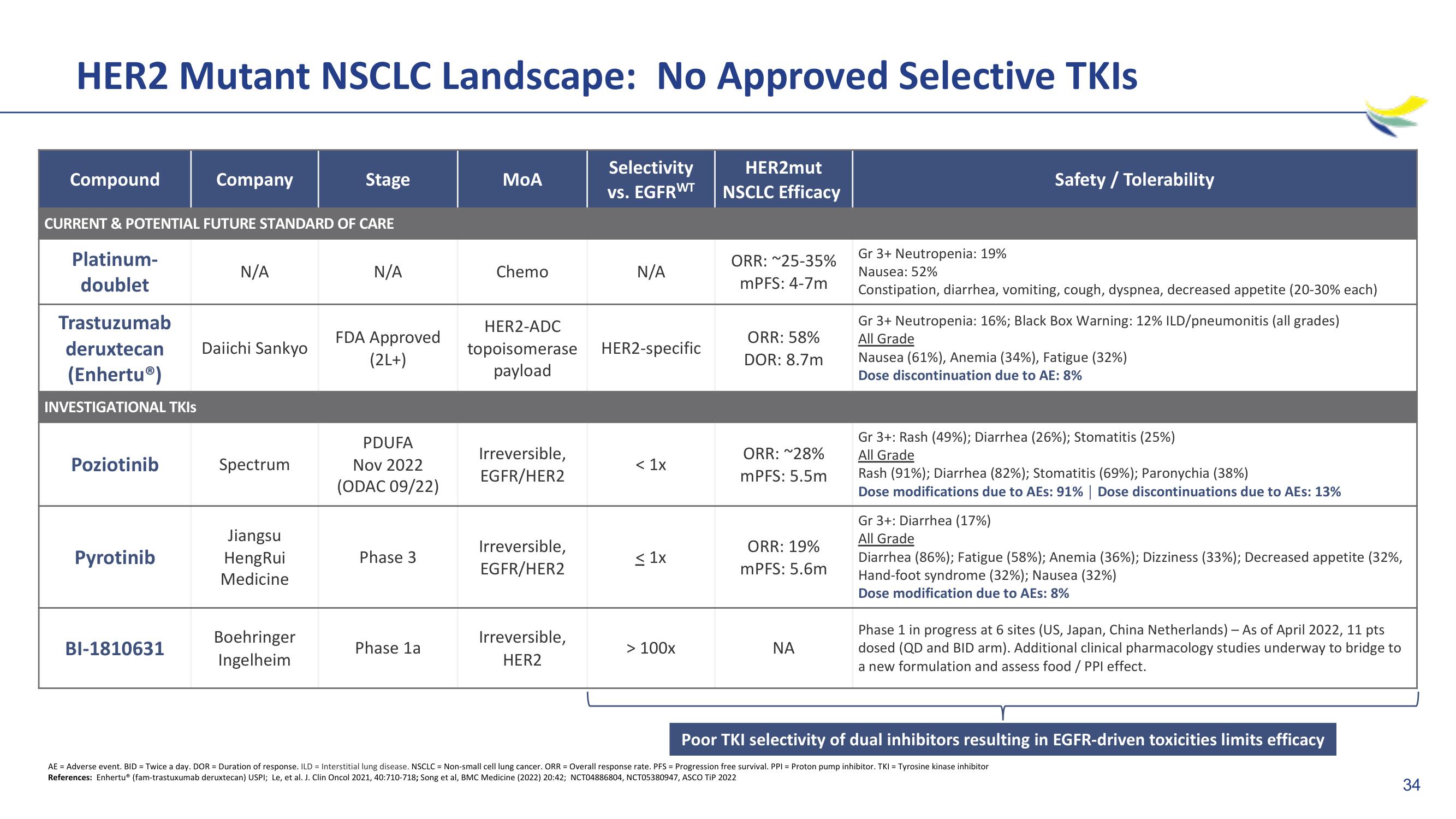
HER2 Mutant NSCLC Landscape: No Approved Selective TKIs
Selectivity
vs. EGFRWT
HER2mut
NSCLC Efficacy
Compound
Platinum-
doublet
CURRENT & POTENTIAL FUTURE STANDARD OF CARE
Poziotinib
Company
Trastuzumab
deruxtecan Daiichi Sankyo
(Enhertu®)
INVESTIGATIONAL TKIS
Pyrotinib
BI-1810631
N/A
Spectrum
Jiangsu
HengRui
Medicine
Stage
Boehringer
Ingelheim
N/A
FDA Approved
(2L+)
PDUFA
Nov 2022
(ODAC 09/22)
Phase 3
Phase 1a
MoA
Chemo
HER2-ADC
topoisomerase
payload
Irreversible,
EGFR/HER2
Irreversible,
EGFR/HER2
Irreversible,
HER2
N/A
HER2-specific
< 1x
≤1x
> 100x
ORR: ~25-35%
mPFS: 4-7m
ORR: 58%
DOR: 8.7m
ORR: ~28%
mPFS: 5.5m
ORR: 19%
mPFS: 5.6m
ΝΑ
Gr 3+ Neutropenia: 19%
Nausea: 52%
Constipation, diarrhea, vomiting, cough, dyspnea, decreased appetite (20-30% each)
Gr 3+ Neutropenia: 16%; Black Box Warning: 12% ILD/pneumonitis (all grades)
All Grade
Nausea (61%), Anemia (34%), Fatigue (32%)
Dose discontinuation due to AE: 8%
Safety / Tolerability
Gr 3+: Rash (49%) ; Diarrhea (26%) ; Stomatitis (25%)
All Grade
Rash (91%); Diarrhea (82%); Stomatitis (69%) ; Paronychia (38%)
Dose modifications due to AEs: 91% | Dose discontinuations due to AEs: 13%
Gr 3+: Diarrhea (17%)
All Grade
Diarrhea (86%); Fatigue (58%) ; Anemia (36%); Dizziness (33%); Decreased appetite (32%,
Hand-foot syndrome (32%) ; Nausea (32%)
Dose modification due to AEs: 8%
Phase 1 in progress at 6 sites (US, Japan, China Netherlands) - As of April 2022, 11 pts
dosed (QD and BID arm). Additional clinical pharmacology studies underway to bridge to
a new formulation and assess food / PPI effect.
Poor TKI selectivity of dual inhibitors resulting in EGFR-driven toxicities limits efficacy
AE = Adverse event. BID = Twice a day. DOR = Duration of response. ILD = Interstitial lung disease. NSCLC = Non-small cell lung cancer. ORR = Overall response rate. PFS = Progression free survival. PPI = Proton pump inhibitor. TKI = Tyrosine kinase inhibitor
References: Enhertu® (fam-trastuxumab deruxtecan) USPI; Le, et al. J. Clin Oncol 2021, 40:710-718; Song et al, BMC Medicine (2022) 20:42; NCT04886804, NCT05380947, ASCO TIP 2022
34View entire presentation